当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Metal-Free, Photocatalytic Method for Aerobic Alkane Iodination
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-15 , DOI: 10.1021/jacs.1c08499 Nathanael A Hirscher 1 , Nidhi Ohri 1 , Qiaomu Yang 1 , Jiawang Zhou 1 , Jessica M Anna 1 , Eric J Schelter 1 , Karen I Goldberg 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-15 , DOI: 10.1021/jacs.1c08499 Nathanael A Hirscher 1 , Nidhi Ohri 1 , Qiaomu Yang 1 , Jiawang Zhou 1 , Jessica M Anna 1 , Eric J Schelter 1 , Karen I Goldberg 1
Affiliation
|
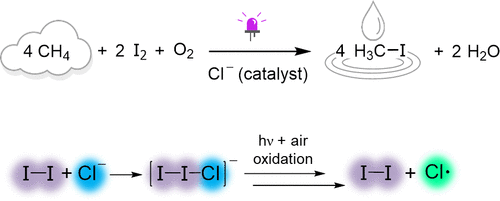
Halogenation is an important alkane functionalization strategy, but O2 is widely considered the most desirable terminal oxidant. Here, the aerobic iodination of alkanes, including methane, was performed using catalytic [nBu4N]Cl and light irradiation (390 nm). Up to 10 turnovers of CH3I were obtained from CH4 and air, using a stop-flow microtubing system. Mechanistic studies using cyclohexane as the substrate revealed important details about the iodination reaction. Iodine (I2) serves multiple roles in the catalysis: (1) as the alkyl radical trap, (2) as a precursor for the light absorber, and (3) as a mediator of aerobic oxidation. The alkane activation is attributed to Cl• derived from photofragmentation of the electron donor–acceptor complex of I2 and Cl–. The kinetic profile of cyclohexane iodination showed that aerobic oxidation of I3– to produce I2 in CH3CN is turnover-limiting.
中文翻译:

一种无金属光催化好氧烷烃碘化方法
卤化是一种重要的烷烃官能化策略,但 O 2被广泛认为是最理想的末端氧化剂。在这里,包括甲烷在内的烷烃的需氧碘化是使用催化 [ n Bu 4 N]Cl 和光照射(390 nm)进行的。使用停流微管系统从 CH 4和空气中获得多达 10 次 CH 3 I 周转。使用环己烷作为底物的机理研究揭示了碘化反应的重要细节。碘 (I 2 ) 在催化中发挥多种作用:(1) 作为烷基自由基捕获剂,(2) 作为光吸收剂的前体,以及 (3) 作为有氧氧化的介质。烷烃活化归因于 Cl•源自I 2和Cl -的电子供体-受体复合物的光裂解。环己烷碘化的动力学曲线表明,有氧氧化 I 3 -在 CH 3 CN 中产生 I 2是周转受限的。
更新日期:2021-11-24
中文翻译:

一种无金属光催化好氧烷烃碘化方法
卤化是一种重要的烷烃官能化策略,但 O 2被广泛认为是最理想的末端氧化剂。在这里,包括甲烷在内的烷烃的需氧碘化是使用催化 [ n Bu 4 N]Cl 和光照射(390 nm)进行的。使用停流微管系统从 CH 4和空气中获得多达 10 次 CH 3 I 周转。使用环己烷作为底物的机理研究揭示了碘化反应的重要细节。碘 (I 2 ) 在催化中发挥多种作用:(1) 作为烷基自由基捕获剂,(2) 作为光吸收剂的前体,以及 (3) 作为有氧氧化的介质。烷烃活化归因于 Cl•源自I 2和Cl -的电子供体-受体复合物的光裂解。环己烷碘化的动力学曲线表明,有氧氧化 I 3 -在 CH 3 CN 中产生 I 2是周转受限的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号