当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enzymatic Cβ–H Functionalization of l-Arg and l-Leu in Nonribosomally Derived Peptidyl Natural Products: A Tale of Two Oxidoreductases
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c08177 Zheng Cui 1 , Han Nguyen 1 , Minakshi Bhardwaj 1 , Xiachang Wang 1 , Martin Büschleb 2 , Anke Lemke 3 , Christian Schütz 3 , Christian Rohrbacher 3 , Pierre Junghanns 3 , Stefan Koppermann 3 , Christian Ducho 3 , Jon S Thorson 1 , Steven G Van Lanen 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c08177 Zheng Cui 1 , Han Nguyen 1 , Minakshi Bhardwaj 1 , Xiachang Wang 1 , Martin Büschleb 2 , Anke Lemke 3 , Christian Schütz 3 , Christian Rohrbacher 3 , Pierre Junghanns 3 , Stefan Koppermann 3 , Christian Ducho 3 , Jon S Thorson 1 , Steven G Van Lanen 1
Affiliation
|
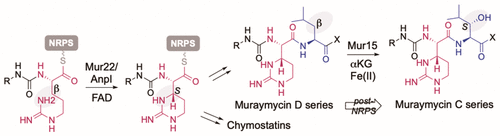
Muraymycins are peptidyl nucleoside antibiotics that contain two Cβ-modified amino acids, (2S,3S)-capreomycidine and (2S,3S)-β-OH-Leu. The former is also a component of chymostatins, which are aldehyde-containing peptidic protease inhibitors that─like muraymycin─are derived from nonribosomal peptide synthetases (NRPSs). Using feeding experiments and in vitro characterization of 12 recombinant proteins, the biosynthetic mechanism for both nonproteinogenic amino acids is now defined. The formation of (2S,3S)-capreomycidine is shown to involve an FAD-dependent dehydrogenase:cyclase that requires an NRPS-bound pathway intermediate as a substrate. This cryptic dehydrogenation strategy is both temporally and mechanistically distinct in comparison to the biosynthesis of other capreomycidine diastereomers, which has previously been shown to proceed by Cβ-hydroxylation of free l-Arg catalyzed by a member of the nonheme Fe2+- and α-ketoglutarate (αKG)-dependent dioxygenase family and (eventually) a dehydration-mediated cyclization process catalyzed by a distinct enzyme(s). Contrary to our initial expectation, the sole nonheme Fe2+- and αKG-dependent dioxygenase candidate Mur15 encoded within the muraymycin gene cluster is instead demonstrated to catalyze specific Cβ hydroxylation of the Leu residue to generate (2S,3S)-β-OH-Leu that is found in most muraymycin congeners. Importantly, and in contrast to known l-Arg-Cβ-hydroxylases, the Mur15-catalyzed reaction occurs after the NRPS-mediated assembly of the peptide scaffold. This late-stage functionalization affords the opportunity to exploit Mur15 as a biocatalyst, proof of concept of which is provided.
中文翻译:

非核糖体衍生的肽基天然产物中 l-Arg 和 l-Leu 的酶促 Cβ-H 功能化:两种氧化还原酶的故事
Muraymycins 是肽基核苷抗生素,含有两个 C β-修饰的氨基酸,(2 S ,3 S )-卷曲霉素和 (2 S ,3 S )-β-OH-Leu。前者也是凝乳抑素的成分,凝乳抑素是一种含醛的肽蛋白酶抑制剂,与胞壁霉素一样,源自非核糖体肽合成酶 (NRPS)。使用 12 种重组蛋白的喂养实验和体外表征,现在定义了两种非蛋白质氨基酸的生物合成机制。(2 S ,3 S)-capreomycidine 显示涉及 FAD 依赖性脱氢酶:需要 NRPS 结合途径中间体作为底物的环化酶。与其他卷曲霉素非对映异构体的生物合成相比,这种隐蔽的脱氢策略在时间上和机制上都是不同的,之前已证明其通过非血红素 Fe 2+ - 和 α 成员催化的游离 l -Arg 的C β -羟基化进行-酮戊二酸 (αKG) 依赖性双加氧酶家族和(最终)由不同酶催化的脱水介导的环化过程。与我们最初的预期相反,唯一的非血红素 Fe 2+- 而在穆雷霉素基因簇中编码的 αKG 依赖性双加氧酶候选物 Mur15 被证明可催化 Leu 残基的特异性 C β羟基化,生成 (2 S ,3 S )-β-OH-Leu,这在大多数穆雷霉素同系物中都有发现。重要的是,与已知的l -Arg-C β -羟化酶相反,Mur15 催化的反应发生在 NRPS 介导的肽支架组装之后。这种后期功能化提供了利用 Mur15 作为生物催化剂的机会,并提供了概念证明。
更新日期:2021-11-24
中文翻译:

非核糖体衍生的肽基天然产物中 l-Arg 和 l-Leu 的酶促 Cβ-H 功能化:两种氧化还原酶的故事
Muraymycins 是肽基核苷抗生素,含有两个 C β-修饰的氨基酸,(2 S ,3 S )-卷曲霉素和 (2 S ,3 S )-β-OH-Leu。前者也是凝乳抑素的成分,凝乳抑素是一种含醛的肽蛋白酶抑制剂,与胞壁霉素一样,源自非核糖体肽合成酶 (NRPS)。使用 12 种重组蛋白的喂养实验和体外表征,现在定义了两种非蛋白质氨基酸的生物合成机制。(2 S ,3 S)-capreomycidine 显示涉及 FAD 依赖性脱氢酶:需要 NRPS 结合途径中间体作为底物的环化酶。与其他卷曲霉素非对映异构体的生物合成相比,这种隐蔽的脱氢策略在时间上和机制上都是不同的,之前已证明其通过非血红素 Fe 2+ - 和 α 成员催化的游离 l -Arg 的C β -羟基化进行-酮戊二酸 (αKG) 依赖性双加氧酶家族和(最终)由不同酶催化的脱水介导的环化过程。与我们最初的预期相反,唯一的非血红素 Fe 2+- 而在穆雷霉素基因簇中编码的 αKG 依赖性双加氧酶候选物 Mur15 被证明可催化 Leu 残基的特异性 C β羟基化,生成 (2 S ,3 S )-β-OH-Leu,这在大多数穆雷霉素同系物中都有发现。重要的是,与已知的l -Arg-C β -羟化酶相反,Mur15 催化的反应发生在 NRPS 介导的肽支架组装之后。这种后期功能化提供了利用 Mur15 作为生物催化剂的机会,并提供了概念证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号