当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Nickel/Photoredox-Catalyzed Deaminative Cross-Coupling of Sterically Hindered Primary Amines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c10150 Julia R Dorsheimer 1 , Melissa A Ashley 1 , Tomislav Rovis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c10150 Julia R Dorsheimer 1 , Melissa A Ashley 1 , Tomislav Rovis 1
Affiliation
|
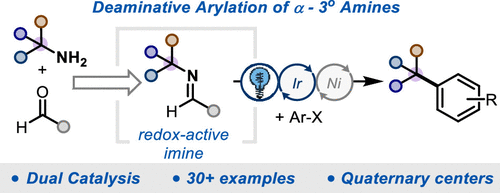
We report a method to activate α-3° amines for deaminative arylation via condensation with an electron-rich aldehyde and merge this reactivity with nickel metallaphotoredox to generate benzylic quaternary centers, a common motif in pharmaceuticals and natural products. The reaction is accelerated by added ammonium salts. Evidence is provided in support of two roles for the additive: inhibition of nickel black formation and acceleration of the overall reaction rate. We demonstrate a robust scope of amine and haloarene coupling partners and show an expedited synthesis of ALK2 inhibitors.
中文翻译:

双镍/光氧化还原催化的空间位阻伯胺的脱氨基交叉偶联
我们报告了一种通过与富电子醛缩合来激活 α-3° 胺以进行脱氨芳基化的方法,并将这种反应性与镍金属光氧化还原结合以生成苄基季铵中心,这是药物和天然产物中的常见基序。加入铵盐可加速反应。提供的证据支持添加剂的两个作用:抑制镍黑形成和加速总反应速率。我们展示了广泛的胺和卤代芳烃偶联伙伴,并展示了 ALK2 抑制剂的加速合成。
更新日期:2021-11-24
中文翻译:

双镍/光氧化还原催化的空间位阻伯胺的脱氨基交叉偶联
我们报告了一种通过与富电子醛缩合来激活 α-3° 胺以进行脱氨芳基化的方法,并将这种反应性与镍金属光氧化还原结合以生成苄基季铵中心,这是药物和天然产物中的常见基序。加入铵盐可加速反应。提供的证据支持添加剂的两个作用:抑制镍黑形成和加速总反应速率。我们展示了广泛的胺和卤代芳烃偶联伙伴,并展示了 ALK2 抑制剂的加速合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号