当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
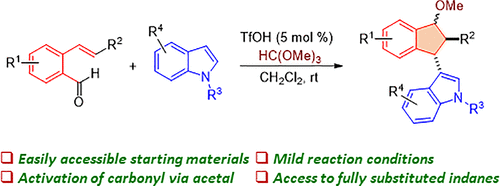
Triflic Acid-Catalyzed Synthesis of Indole-Substituted Indane Derivatives via In Situ Formed Acetal-Facilitated Nucleophilic Addition and 4π-Electron-5-Carbon Electrocyclization Sequence
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-11 , DOI: 10.1021/acs.joc.1c01396 Golla Ramesh 1 , Rengarajan Balamurugan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-11 , DOI: 10.1021/acs.joc.1c01396 Golla Ramesh 1 , Rengarajan Balamurugan 1
Affiliation
|
An efficient protocol for the synthesis of indole-substituted indanes from o-alkenylbenzaldehydes under acetalization conditions has been presented. The cyclization occurs via a nucleophilic addition of indole on the oxacarbenium ion generated from acetal formed under the reaction condition followed by a conrotatory 4π-electrocyclization reaction, which takes care of the exclusive diastereoselectivity observed during the cyclization step. Olefin geometry of o-alkenylbenzaldehyde and the amount of indole play a decisive role in the success of this cyclization process.
中文翻译:

三氟甲磺酸催化合成吲哚取代的茚满衍生物通过原位形成的乙缩醛促进亲核加成和4π-电子-5-碳电环化序列
提出了一种在缩醛化条件下从邻烯基苯甲醛合成吲哚取代茚满的有效方案。环化是通过在反应条件下由缩醛生成的氧杂碳鎓离子上亲核加成吲哚发生的,然后是旋转 4π-电环化反应,它负责在环化步骤中观察到的独特的非对映选择性。邻烯基苯甲醛的烯烃几何结构和吲哚的量在该环化过程的成功中起决定性作用。
更新日期:2021-12-03
中文翻译:

三氟甲磺酸催化合成吲哚取代的茚满衍生物通过原位形成的乙缩醛促进亲核加成和4π-电子-5-碳电环化序列
提出了一种在缩醛化条件下从邻烯基苯甲醛合成吲哚取代茚满的有效方案。环化是通过在反应条件下由缩醛生成的氧杂碳鎓离子上亲核加成吲哚发生的,然后是旋转 4π-电环化反应,它负责在环化步骤中观察到的独特的非对映选择性。邻烯基苯甲醛的烯烃几何结构和吲哚的量在该环化过程的成功中起决定性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号