当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bidirectional Total Synthesis of Phainanoid A via Strategic Use of Ketones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c11117 Jiaxin Xie 1 , Xin Liu 1 , Nan Zhang 1 , Shinyoung Choi 1 , Guangbin Dong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-12 , DOI: 10.1021/jacs.1c11117 Jiaxin Xie 1 , Xin Liu 1 , Nan Zhang 1 , Shinyoung Choi 1 , Guangbin Dong 1
Affiliation
|
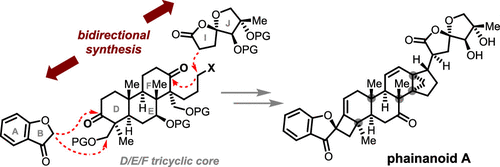
Here we report the total synthesis of phainanoid A, a unique dammarane-type triterpenoid (DTT), using an unusual bidirectional synthetic strategy. It features two transition-metal-mediated highly diastereoselective transformations to access the two challenging strained ring systems that branch toward opposite directions from the tricyclic core. This work also highlights the strategic use of ketones (or enol triflates) as versatile handles for rapid growth of molecular complexity in all key transformations, which paves the way for efficient preparations of complex and biologically significant DTTs.
中文翻译:

通过酮的战略使用双向全合成 Phananoid A
在这里,我们报告了 phainanoid A 的全合成,这是一种独特的达玛烷型三萜 (DTT),使用不寻常的双向合成策略。它具有两个过渡金属介导的高度非对映选择性转化,以访问从三环核心向相反方向分支的两个具有挑战性的应变环系统。这项工作还强调了酮(或烯醇三氟甲磺酸酯)作为通用手柄的战略用途,可在所有关键转化中快速增加分子复杂性,这为有效制备复杂且具有生物学意义的 DTT 铺平了道路。
更新日期:2021-11-24
中文翻译:

通过酮的战略使用双向全合成 Phananoid A
在这里,我们报告了 phainanoid A 的全合成,这是一种独特的达玛烷型三萜 (DTT),使用不寻常的双向合成策略。它具有两个过渡金属介导的高度非对映选择性转化,以访问从三环核心向相反方向分支的两个具有挑战性的应变环系统。这项工作还强调了酮(或烯醇三氟甲磺酸酯)作为通用手柄的战略用途,可在所有关键转化中快速增加分子复杂性,这为有效制备复杂且具有生物学意义的 DTT 铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号