当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proximity-Dependent Labeling of Cysteines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-11 , DOI: 10.1021/jacs.1c07069 Sudeshna Sen 1, 2 , Nadia Sultana 1, 3 , Scott A Shaffer 1, 3 , Paul R Thompson 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-11 , DOI: 10.1021/jacs.1c07069 Sudeshna Sen 1, 2 , Nadia Sultana 1, 3 , Scott A Shaffer 1, 3 , Paul R Thompson 1, 2
Affiliation
|
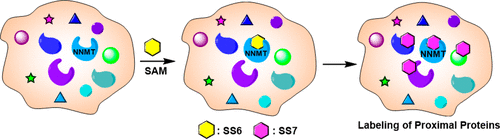
Mapping protein–protein interactions is crucial for understanding various signaling pathways in living cells, and developing new techniques for this purpose has attracted significant interest. Classic methods (e.g., the yeast two-hybrid) have been supplanted by more sophisticated chemical approaches that label proximal proteins (e.g., BioID, APEX). Herein we describe a proximity-based approach that uniquely labels cysteines. Our approach exploits the nicotinamide N-methyltransferase (NNMT)-catalyzed methylation of an alkyne-substituted 4-chloropyridine (SS6). Upon methylation of the pyridinium nitrogen, this latent electrophile diffuses out of the active site and labels proximal proteins on short time scales (≤5 min). We validated this approach by identifying known (and novel) interacting partners of protein arginine deiminase 2 (PAD2) and pyruvate dehydrogenase kinase 1 (PDK1). To our knowledge, this technology uniquely exploits a suicide substrate to label proximal cysteines in live cells.
中文翻译:

半胱氨酸的邻近依赖性标记
绘制蛋白质-蛋白质相互作用对于了解活细胞中的各种信号通路至关重要,为此开发新技术已引起了极大的兴趣。经典方法(例如,酵母双杂交法)已被更复杂的标记近端蛋白质的化学方法(例如,BioID、APEX)所取代。在这里,我们描述了一种基于邻近度的方法,该方法可以唯一地标记半胱氨酸。我们的方法利用烟酰胺N-甲基转移酶 (NNMT) 催化的炔烃取代的 4-氯吡啶 ( SS6 ) 甲基化)。在吡啶鎓氮甲基化后,这种潜在的亲电试剂会扩散出活性位点,并在短时间内(≤5 分钟)标记近端蛋白质。我们通过识别蛋白质精氨酸脱亚胺酶 2 (PAD2) 和丙酮酸脱氢酶激酶 1 (PDK1) 的已知(和新型)相互作用伙伴来验证这种方法。据我们所知,这项技术独特地利用了一种自杀底物来标记活细胞中的近端半胱氨酸。
更新日期:2021-11-24
中文翻译:

半胱氨酸的邻近依赖性标记
绘制蛋白质-蛋白质相互作用对于了解活细胞中的各种信号通路至关重要,为此开发新技术已引起了极大的兴趣。经典方法(例如,酵母双杂交法)已被更复杂的标记近端蛋白质的化学方法(例如,BioID、APEX)所取代。在这里,我们描述了一种基于邻近度的方法,该方法可以唯一地标记半胱氨酸。我们的方法利用烟酰胺N-甲基转移酶 (NNMT) 催化的炔烃取代的 4-氯吡啶 ( SS6 ) 甲基化)。在吡啶鎓氮甲基化后,这种潜在的亲电试剂会扩散出活性位点,并在短时间内(≤5 分钟)标记近端蛋白质。我们通过识别蛋白质精氨酸脱亚胺酶 2 (PAD2) 和丙酮酸脱氢酶激酶 1 (PDK1) 的已知(和新型)相互作用伙伴来验证这种方法。据我们所知,这项技术独特地利用了一种自杀底物来标记活细胞中的近端半胱氨酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号