当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
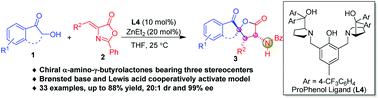
Dinuclear zinc-catalyzed asymmetric [3 + 2] cyclization reaction for direct assembly of chiral α-amino-γ-butyrolactones bearing three stereocenters
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-06 , DOI: 10.1039/d1qo01338f Wen-Peng Yang 1 , Shi-Kun Jia 1 , Tian-Tian Liu 1 , Yuan-Zhao Hua 1 , Min-Can Wang 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-06 , DOI: 10.1039/d1qo01338f Wen-Peng Yang 1 , Shi-Kun Jia 1 , Tian-Tian Liu 1 , Yuan-Zhao Hua 1 , Min-Can Wang 1
Affiliation
|
An efficient enantioselective [3 + 2] annulation of α-hydroxy-1-indanones and alkylidene azlactones has been developed with chiral dinuclear zinc catalysts via a Brønsted base and Lewis acid cooperative activation model. This practical methodology gives access to a broad range of chiral α-amino-γ-butyrolactones bearing three stereocenters in good yields with excellent diastereo- and enatiostereoselectivities (up to 88% yield, 20 : 1 dr, 99% ee). This transformation features broad functional group tolerance, gram-scale synthesis, and further prolongation with α-hydroxyacetophenone or 3-hydroxychroman-4-one.
中文翻译:

双核锌催化不对称 [3 + 2] 环化反应,用于直接组装带有三个立体中心的手性 α-氨基-γ-丁内酯
通过布朗斯台德碱和路易斯酸协同活化模型,使用手性双核锌催化剂开发了 α-羟基-1-茚满酮和亚烷基吖内酯的有效对映选择性 [3 + 2] 环化。这种实用的方法可以获得范围广泛的具有三个立体中心的手性 α-氨基-γ-丁内酯,收率良好,具有出色的非对映选择性和对映立体选择性(收率高达 88%,20:1 dr,99% ee)。这种转化具有广泛的官能团耐受性、克级合成以及与 α-羟基苯乙酮或 3-羟基色满-4-one 的进一步延长。
更新日期:2021-11-11
中文翻译:

双核锌催化不对称 [3 + 2] 环化反应,用于直接组装带有三个立体中心的手性 α-氨基-γ-丁内酯
通过布朗斯台德碱和路易斯酸协同活化模型,使用手性双核锌催化剂开发了 α-羟基-1-茚满酮和亚烷基吖内酯的有效对映选择性 [3 + 2] 环化。这种实用的方法可以获得范围广泛的具有三个立体中心的手性 α-氨基-γ-丁内酯,收率良好,具有出色的非对映选择性和对映立体选择性(收率高达 88%,20:1 dr,99% ee)。这种转化具有广泛的官能团耐受性、克级合成以及与 α-羟基苯乙酮或 3-羟基色满-4-one 的进一步延长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号