当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to azonanes via Pd-catalyzed decarboxylative [5 + 4] cycloaddition with exclusive regioselectivity
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-30 , DOI: 10.1039/d1qo01405f Yin Liu 1, 2 , Yicheng He 2 , Yang Liu 2 , Kun Wei 1 , Wusheng Guo 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-30 , DOI: 10.1039/d1qo01405f Yin Liu 1, 2 , Yicheng He 2 , Yang Liu 2 , Kun Wei 1 , Wusheng Guo 2, 3
Affiliation
|
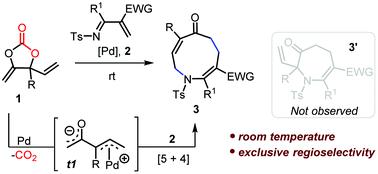
A highly efficient approach for the synthesis of azonanes via Pd-catalyzed decarboxylative [5 + 4] cycloaddition has been developed. This procedure utilizes methylene cyclic carbonates as a C5-dipole and 1-azadiene as a C4-synthon. The reactions could be performed at room temperature. The protocol features wide functional group tolerance with exclusive regioselectivity and generates CO2 as the only byproduct.
中文翻译:

通过具有独特区域选择性的 Pd 催化脱羧 [5 + 4] 环加成反应获得氮烷
已经开发了一种通过Pd 催化脱羧 [5 + 4] 环加成合成氮烷的高效方法。该程序使用亚甲基环状碳酸酯作为 C5-偶极子和 1-氮杂二烯作为 C4-合成子。反应可以在室温下进行。该协议具有广泛的官能团耐受性和独特的区域选择性,并产生 CO 2作为唯一的副产品。
更新日期:2021-11-11
中文翻译:

通过具有独特区域选择性的 Pd 催化脱羧 [5 + 4] 环加成反应获得氮烷
已经开发了一种通过Pd 催化脱羧 [5 + 4] 环加成合成氮烷的高效方法。该程序使用亚甲基环状碳酸酯作为 C5-偶极子和 1-氮杂二烯作为 C4-合成子。反应可以在室温下进行。该协议具有广泛的官能团耐受性和独特的区域选择性,并产生 CO 2作为唯一的副产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号