Medical Image Analysis ( IF 10.7 ) Pub Date : 2021-11-11 , DOI: 10.1016/j.media.2021.102304 Junhao Wen 1 , Erdem Varol 2 , Aristeidis Sotiras 3 , Zhijian Yang 1 , Ganesh B Chand 4 , Guray Erus 1 , Haochang Shou 5 , Ahmed Abdulkadir 1 , Gyujoon Hwang 1 , Dominic B Dwyer 6 , Alessandro Pigoni 7 , Paola Dazzan 8 , Rene S Kahn 9 , Hugo G Schnack 10 , Marcus V Zanetti 11 , Eva Meisenzahl 12 , Geraldo F Busatto 11 , Benedicto Crespo-Facorro 13 , Romero-Garcia Rafael 14 , Christos Pantelis 15 , Stephen J Wood 16 , Chuanjun Zhuo 17 , Russell T Shinohara 5 , Yong Fan 1 , Ruben C Gur 18 , Raquel E Gur 18 , Theodore D Satterthwaite 19 , Nikolaos Koutsouleris 6 , Daniel H Wolf 19 , Christos Davatzikos 1 ,
|
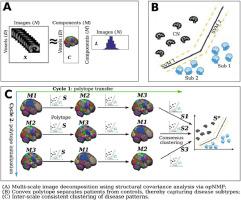
Disease heterogeneity is a significant obstacle to understanding pathological processes and delivering precision diagnostics and treatment. Clustering methods have gained popularity for stratifying patients into subpopulations (i.e., subtypes) of brain diseases using imaging data. However, unsupervised clustering approaches are often confounded by anatomical and functional variations not related to a disease or pathology of interest. Semi-supervised clustering techniques have been proposed to overcome this and, therefore, capture disease-specific patterns more effectively. An additional limitation of both unsupervised and semi-supervised conventional machine learning methods is that they typically model, learn and infer from data using a basis of feature sets pre-defined at a fixed anatomical or functional scale (e.g., atlas-based regions of interest). Herein we propose a novel method, “Multi-scAle heteroGeneity analysIs and Clustering” (MAGIC), to depict the multi-scale presentation of disease heterogeneity, which builds on a previously proposed semi-supervised clustering method, HYDRA. It derives multi-scale and clinically interpretable feature representations and exploits a double-cyclic optimization procedure to effectively drive identification of inter-scale-consistent disease subtypes. More importantly, to understand the conditions under which the clustering model can estimate true heterogeneity related to diseases, we conducted extensive and systematic semi-simulated experiments to evaluate the proposed method on a sizeable healthy control sample from the UK Biobank (N = 4403). We then applied MAGIC to imaging data from Alzheimer's disease (ADNI, N = 1728) and schizophrenia (PHENOM, N = 1166) patients to demonstrate its potential and challenges in dissecting the neuroanatomical heterogeneity of common brain diseases. Taken together, we aim to provide guidance regarding when such analyses can succeed or should be taken with caution. The code of the proposed method is publicly available at https://github.com/anbai106/MAGIC.
中文翻译:

脑图像的多尺度半监督聚类:推导疾病亚型
疾病异质性是理解病理过程和提供精确诊断和治疗的重大障碍。使用成像数据将患者分层为脑部疾病亚群(即亚型)的聚类方法已经流行。然而,无监督聚类方法经常被与感兴趣的疾病或病理无关的解剖和功能变化所混淆。半监督聚类技术已经被提出来克服这个问题,从而更有效地捕获疾病特定的模式。无监督和半监督传统机器学习方法的另一个限制是,它们通常使用以固定解剖或功能尺度预定义的特征集为基础(例如,基于图集的感兴趣区域)来建模、学习和推断数据。 )。在此,我们提出了一种新方法“多尺度异质性分析和聚类”(MAGIC),用于描述疾病异质性的多尺度呈现,该方法建立在先前提出的半监督聚类方法 HYDRA 的基础上。它导出多尺度和临床可解释的特征表示,并利用双循环优化程序来有效地驱动尺度间一致的疾病亚型的识别。更重要的是,为了了解聚类模型可以估计与疾病相关的真实异质性的条件,我们进行了广泛且系统的半模拟实验,以在英国生物银行( N = 4403)的大量健康对照样本上评估所提出的方法。 然后,我们将 MAGIC 应用于阿尔茨海默氏病 (ADNI, N = 1728) 和精神分裂症 (PHENOM, N = 1166) 患者的成像数据,以证明其在剖析常见脑部疾病的神经解剖异质性方面的潜力和挑战。总而言之,我们的目标是就此类分析何时能够成功或何时应谨慎进行提供指导。所提出方法的代码可在 https://github.com/anbai106/MAGIC 上公开获取。











































 京公网安备 11010802027423号
京公网安备 11010802027423号