Cell Stem Cell ( IF 19.8 ) Pub Date : 2021-11-10 , DOI: 10.1016/j.stem.2021.10.009 Vaibhao Janbandhu 1 , Vikram Tallapragada 1 , Ralph Patrick 1 , Yanzhen Li 2 , Dhanushi Abeygunawardena 3 , David T Humphreys 1 , Ella M M A Martin 4 , Alexander O Ward 1 , Osvaldo Contreras 1 , Nona Farbehi 5 , Ernestene Yao 4 , Junjie Du 4 , Sally L Dunwoodie 1 , Nenad Bursac 6 , Richard P Harvey 7
|
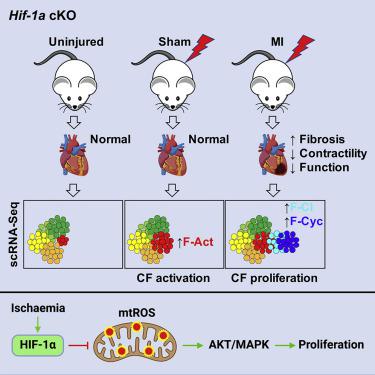
We report that cardiac fibroblasts (CFs) and mesenchymal progenitors are more hypoxic than other cardiac interstitial populations, express more hypoxia-inducible factor 1α (HIF-1α), and exhibit increased glycolytic metabolism. CF-specific deletion of Hif-1a resulted in decreased HIF-1 target gene expression and increased mesenchymal progenitors in uninjured hearts and increased CF activation without proliferation following sham injury, as demonstrated using single-cell RNA sequencing (scRNA-seq). After myocardial infarction (MI), however, there was ∼50% increased CF proliferation and excessive scarring and contractile dysfunction, a scenario replicated in 3D engineered cardiac microtissues. CF proliferation was associated with higher reactive oxygen species (ROS) as occurred also in wild-type mice treated with the mitochondrial ROS generator MitoParaquat (MitoPQ). The mitochondrial-targeted antioxidant MitoTEMPO rescued Hif-1a mutant phenotypes. Thus, HIF-1α in CFs provides a critical braking mechanism against excessive post-ischemic CF activation and proliferation through regulation of mitochondrial ROS. CFs are potential cellular targets for designer antioxidant therapies in cardiovascular disease.
中文翻译:

Hif-1a 抑制心肌梗死后 ROS 诱导的心肌成纤维细胞增殖
我们报告说,心脏成纤维细胞 (CF) 和间充质祖细胞比其他心脏间质细胞群更缺氧,表达更多的缺氧诱导因子 1α (HIF-1α),并表现出糖酵解代谢增加。CF 特异性删除Hif-1a导致未受伤心脏中 HIF-1 靶基因表达减少和间充质祖细胞增加,假损伤后 CF 激活增加而无增殖,如使用单细胞 RNA 测序 (scRNA-seq) 所证明的。然而,心肌梗死 (MI) 后,CF 增殖增加了约 50%,瘢痕形成过度,收缩功能障碍也增加了,这种情况在 3D 工程心脏微组织中得到重现。CF 增殖与较高的活性氧 (ROS) 相关,这也发生在用线粒体 ROS 生成剂 MitoPQ (MitoPQ) 处理的野生型小鼠中。线粒体靶向抗氧化剂 MitoTEMPO 拯救了Hif-1a突变表型。因此,CF 中的 HIF-1α 通过调节线粒体 ROS 提供了一种关键的制动机制,可防止过度缺血后 CF 激活和增殖。CF 是心血管疾病抗氧化疗法设计者的潜在细胞靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号