当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of 6H-benzo[c]thiochromenes via a tandem reaction of arynes with thionoesters
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-10 , DOI: 10.1039/d1qo01177d Yi An 1 , Fang Zhang 1 , Guangfen Du 1 , Zhihua Cai 1 , Lin He 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-10 , DOI: 10.1039/d1qo01177d Yi An 1 , Fang Zhang 1 , Guangfen Du 1 , Zhihua Cai 1 , Lin He 1
Affiliation
|
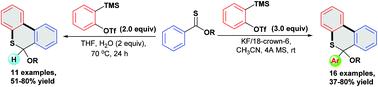
A mild and transition-metal free tandem reaction for the construction of 6H-benzo[c]thiochromenes has been developed. Thionoesters react with two molecules of arynes through a Diels–Alder cycloaddition/nucleophilic arylation process to afford 6-alkoxy-6-aryl-6H-benzo[c]thiochromenes in 37–80% yields. In addition, the Diels–Alder cycloaddition/aromatization tandem reaction of thionoesters with one molecule of aryne provides the construction of 6-alkoxyl-6H-benzo[c]thiochromenes in 51–75% yields.
中文翻译:

通过芳烃与硫酮酯的串联反应构建 6H-苯并[c] 硫代色烯
已开发出一种温和且无过渡金属的串联反应,用于构建 6 H-苯并[ c ] 硫色素烯。硫代酯通过狄尔斯-阿尔德环加成/亲核芳基化过程与两分子芳烃反应,以 37-80% 的产率得到 6-烷氧基-6-芳基-6 H-苯并[ c ] 硫色烯。此外,硫酮酯与一分子芳烃的 Diels-Alder 环加成/芳构化串联反应以 51-75% 的产率构建了 6-alkoxyl-6 H -benzo [ c ]thiochromenes。
更新日期:2021-11-10
中文翻译:

通过芳烃与硫酮酯的串联反应构建 6H-苯并[c] 硫代色烯
已开发出一种温和且无过渡金属的串联反应,用于构建 6 H-苯并[ c ] 硫色素烯。硫代酯通过狄尔斯-阿尔德环加成/亲核芳基化过程与两分子芳烃反应,以 37-80% 的产率得到 6-烷氧基-6-芳基-6 H-苯并[ c ] 硫色烯。此外,硫酮酯与一分子芳烃的 Diels-Alder 环加成/芳构化串联反应以 51-75% 的产率构建了 6-alkoxyl-6 H -benzo [ c ]thiochromenes。











































 京公网安备 11010802027423号
京公网安备 11010802027423号