Water Research ( IF 11.4 ) Pub Date : 2021-11-09 , DOI: 10.1016/j.watres.2021.117854 Guoguang Wang 1 , Yu Liu 2 , Xu Wang 1 , Xu Dong 1 , Na Jiang 1 , Haixia Wang 3
|
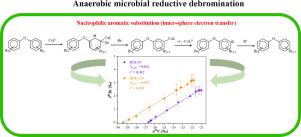
Polybrominated diphenyl ethers (PBDEs), one kind of persistent organic pollutants, were widely detected in coastal wetlands. Microbial reductive debromination is one of the most important attenuation processes for PBDEs in anaerobic environment, whereas the underlying reaction mechanisms remain elusive. Dual-element stable isotope analysis was recently recognized to distinguish different reaction mechanism for degradation of organic pollutants. In this study, the dual carbon-bromine isotope effects associated with the anaerobic microbial degradation were first investigated to characterize the reaction mechanisms for BDE-47 and BDE-153. Presence of lower brominated congeners indicated stepwise debromination as the main degradation pathway, with the preferential removal of bromine in para position > meta/ortho position. The pronounced isotope fractionation was observed for both carbon and bromine, with similar carbon (εC) and bromine isotope enrichment factor (εBr) between BDE-47 (εC = -5.98‰, εBr = -2.44‰) and BDE-153 (εC = -5.57‰, εBr = -2.06‰) during the microbial degradation. Compared to εC and εBr, the correlation of carbon and isotope effects (ΛC/Br = Δδ81Br/Δδ13C) was almost the same between BDE-47 (0.436) and BDE-153 (0.435), indicating the similar reaction mechanism. The calculated carbon and bromine apparent kinetic isotope effects (AKIEC and AKIEBr) were 1.0773 and 1.0098 for BDE-47 and 1.0716 and 1.0125 for BDE-153, within range reported for degradation of halogenated compounds following nucleophilic substitution. Combination analysis of degradation products, ΛC/Br and AKIE, all the results pointed to that the anaerobic reductive debromination of BDE-47 and BDE-153 followed the nucleophilic aromatic substitution, with the addition of cofactor to the benzene ring concomitant with dissociation of carbon-bromine bond via the inner-sphere electron transfer, and the cleavage of C-Br bond was the rate-determining step. This study contributed to the development of dual carbon-bromine isotope analysis as a robust approach to probe the fate of PBDEs in contaminated sites.
中文翻译:

应用碳溴双稳定同位素分析表征湿地底水中多溴二苯醚的厌氧微降解机制
多溴联苯醚(PBDEs)是一种持久性有机污染物,在沿海湿地中被广泛检测到。微生物还原脱溴是厌氧环境中多溴二苯醚最重要的衰减过程之一,而潜在的反应机制仍然难以捉摸。最近认识到双元素稳定同位素分析可以区分降解有机污染物的不同反应机制。在这项研究中,首先研究了与厌氧微生物降解相关的双碳溴同位素效应,以表征 BDE-47 和 BDE-153 的反应机制。低溴化同源物的存在表明逐步脱溴是主要的降解途径,优先去除对位 > 间位/邻位的溴。ε C ) 和BDE-47 ( ε C = -5.98‰, ε Br = -2.44‰) 和 BDE-153 ( ε C = -5.57‰, ε Br = -2.06‰)之间的溴同位素富集因子 (ε Br )在微生物降解过程中。与ε C和ε Br相比,BDE-47 (0.436) 和 BDE-153 (0.435)的碳和同位素效应的相关性 (Λ C/Br = Δ δ 81 Br/Δ δ 13 C) 几乎相同,表明类似的反应机理。计算的碳和溴表观动力学同位素效应(AKIEC和AKIE Br ) 对于 BDE-47 为 1.0773 和 1.0098,对于 BDE-153 为 1.0716 和 1.0125,在报告的亲核取代后卤化化合物降解的范围内。对降解产物Λ C/Br和AKIE 的组合分析,所有结果都表明BDE-47 和BDE-153 的厌氧还原脱溴发生在亲核芳香取代之后,辅因子添加到苯环伴随着BDE-47 和BDE-153 的解离碳-溴键通过内球电子转移,C-Br键的断裂是速率决定步骤。这项研究有助于开发双碳溴同位素分析,作为探测污染场地中多溴二苯醚归宿的有效方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号