当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Quinazoline-2,4(1H,3H)-dione Derivatives Containing 3-Substituted Piperizines as Potent PARP-1/2 Inhibitors─Design, Synthesis, In Vivo Antitumor Activity, and X-ray Crystal Structure Analysis
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-08 , DOI: 10.1021/acs.jmedchem.1c01522 Jie Zhou 1 , Ming Ji 2, 3 , Xiaoyu Wang 1 , Hailong Zhao 1 , Ran Cao 1 , Jing Jin 2, 3 , Yan Li 4 , Xianhong Chen 4, 5 , Li Sheng 4 , Xiaoguang Chen 2, 3 , Bailing Xu 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-08 , DOI: 10.1021/acs.jmedchem.1c01522 Jie Zhou 1 , Ming Ji 2, 3 , Xiaoyu Wang 1 , Hailong Zhao 1 , Ran Cao 1 , Jing Jin 2, 3 , Yan Li 4 , Xianhong Chen 4, 5 , Li Sheng 4 , Xiaoguang Chen 2, 3 , Bailing Xu 1
Affiliation
|
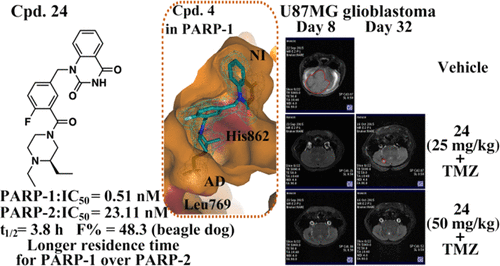
Inhibiting PARP-1/2 offered an important arsenal for cancer treatments via interfering with DNA repair of cancer cells. Novel PARP-1/2 inhibitors were designed by capitalizing on methyl- or ethyl-substituted piperizine ring to capture the characteristics of adenine-ribose binding site (AD site), and their unique binding features were revealed by the cocrystal structures of compounds 4 and 6 in PARP-1. The investigation on structure–activity relationship resulted in compounds 24 and 32 with high enzymatic potency, binding selectivity, and significantly longer residence time for PARP-1 over PARP-2 (compound 24, PARP-1: IC50 = 0.51 nM, PARP-2: IC50 = 23.11 nM; compound 32, PARP-1: IC50 = 1.31 nM, PARP-2: IC50 = 15.63 nM). Furthermore, compound 24 was determined to be an attractive candidate molecule, which possessed an acceptable pharmacokinetic profile and produced remarkable antitumor activity in both breast cancer xenograft model and glioblastoma orthotopic model in mice, either alone or in combination treatment.
中文翻译:

发现含有 3-取代哌嗪类作为 PARP-1/2 强抑制剂的喹唑啉-2,4(1H,3H)-二酮衍生物─设计、合成、体内抗肿瘤活性和 X 射线晶体结构分析
抑制 PARP-1/2 通过干扰癌细胞的 DNA 修复为癌症治疗提供了重要的武器库。新型 PARP-1/2 抑制剂是通过利用甲基或乙基取代的哌嗪环来捕获腺嘌呤-核糖结合位点 (AD 位点) 的特征而设计的,其独特的结合特征通过化合物4的共晶结构和PARP-1 中的6个。对结构-活性关系的研究导致化合物24和32对 PARP-1 的酶效价、结合选择性和比 PARP-2 显着更长的停留时间(化合物24,PARP-1:IC 50 = 0.51 nM,PARP- 2:集成电路50= 23.11 纳米; 化合物32,PARP-1:IC 50 = 1.31 nM,PARP-2:IC 50 = 15.63 nM)。此外,化合物24被确定为一种有吸引力的候选分子,其具有可接受的药代动力学特征,并在单独或联合治疗的小鼠乳腺癌异种移植模型和胶质母细胞瘤原位模型中产生显着的抗肿瘤活性。
更新日期:2021-11-25
中文翻译:

发现含有 3-取代哌嗪类作为 PARP-1/2 强抑制剂的喹唑啉-2,4(1H,3H)-二酮衍生物─设计、合成、体内抗肿瘤活性和 X 射线晶体结构分析
抑制 PARP-1/2 通过干扰癌细胞的 DNA 修复为癌症治疗提供了重要的武器库。新型 PARP-1/2 抑制剂是通过利用甲基或乙基取代的哌嗪环来捕获腺嘌呤-核糖结合位点 (AD 位点) 的特征而设计的,其独特的结合特征通过化合物4的共晶结构和PARP-1 中的6个。对结构-活性关系的研究导致化合物24和32对 PARP-1 的酶效价、结合选择性和比 PARP-2 显着更长的停留时间(化合物24,PARP-1:IC 50 = 0.51 nM,PARP- 2:集成电路50= 23.11 纳米; 化合物32,PARP-1:IC 50 = 1.31 nM,PARP-2:IC 50 = 15.63 nM)。此外,化合物24被确定为一种有吸引力的候选分子,其具有可接受的药代动力学特征,并在单独或联合治疗的小鼠乳腺癌异种移植模型和胶质母细胞瘤原位模型中产生显着的抗肿瘤活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号