当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free electrochemical synthesis of α-ketoamides via decarboxylative coupling of α-keto acids with isocyanides and water
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-26 , DOI: 10.1039/d1qo01351c Feng Zhao 1 , Na Meng 2 , Ting Sun 1 , Jiangwei Wen 2 , Xiaohui Zhao 3 , Wei Wei 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-26 , DOI: 10.1039/d1qo01351c Feng Zhao 1 , Na Meng 2 , Ting Sun 1 , Jiangwei Wen 2 , Xiaohui Zhao 3 , Wei Wei 2, 3
Affiliation
|
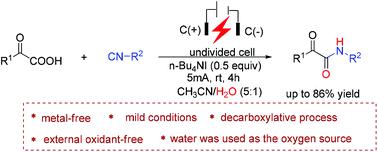
A mild and environmentally benign electrochemical strategy has been developed for the synthesis of α-ketoamides via a decarboxylative coupling reaction of α-keto acids with isocyanides and water. The present reaction was efficiently conducted under constant current electrolysis using n-Bu4NI as the electrolyte in an undivided cell. A series of α-ketoamides could be conveniently and rapidly constructed at room temperature in the absence of transition metal catalysts and external oxidants. Mechanistic studies suggested that water not only served as a starting material to construct α-ketoamides, but also played an important role in promoting the electrochemical redox of α-keto acids.
中文翻译:

通过α-酮酸与异氰化物和水的脱羧偶联无金属电化学合成α-酮酰胺
通过α-酮酸与异氰化物和水的脱羧偶联反应,已经开发出一种温和且环境友好的电化学策略,用于合成α-酮酰胺。本反应在恒流电解下有效地进行,使用n -Bu 4 NI 作为未分隔电池中的电解质。在没有过渡金属催化剂和外部氧化剂的情况下,可以在室温下方便、快速地构建一系列α-酮酰胺。机理研究表明,水不仅可以作为构建 α-酮酰胺的起始材料,而且在促进 α-酮酸的电化学氧化还原方面也发挥了重要作用。
更新日期:2021-11-08
中文翻译:

通过α-酮酸与异氰化物和水的脱羧偶联无金属电化学合成α-酮酰胺
通过α-酮酸与异氰化物和水的脱羧偶联反应,已经开发出一种温和且环境友好的电化学策略,用于合成α-酮酰胺。本反应在恒流电解下有效地进行,使用n -Bu 4 NI 作为未分隔电池中的电解质。在没有过渡金属催化剂和外部氧化剂的情况下,可以在室温下方便、快速地构建一系列α-酮酰胺。机理研究表明,水不仅可以作为构建 α-酮酰胺的起始材料,而且在促进 α-酮酸的电化学氧化还原方面也发挥了重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号