JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2021-11-06 , DOI: 10.1016/j.jcin.2021.10.018 Firas Zahr 1 , Howard K Song 1 , Scott M Chadderdon 1 , Hemal Gada 2 , Mubashir Mumtaz 2 , Timothy Byrne 3 , Merick Kirshner 3 , Tanvir Bajwa 4 , Eric Weiss 4 , Susheel Kodali 5 , Isaac George 5 , John Heiser 6 , William M Merhi 6 , Jeremy J Thaden 7 , Angie Zhang 8 , D Scott Lim 9 , Michael J Reardon 10 , David H Adams 11 , Michael J Mack 12 , Martin B Leon 5
|
Objectives
The aim of this study was to evaluate outcomes of transcatheter mitral valve replacement (TMVR) with transfemoral access in patients at prohibitive or high surgical risk.
Background
Prohibitive surgical risk may preclude mitral valve replacement surgery in some patients. The investigational Intrepid TMVR system has previously been evaluated using transapical access for delivery of a self-expanding bioprosthetic valve.
Methods
This prospective, multicenter, nonrandomized early feasibility study evaluated the safety and performance of the Intrepid valve using transfemoral access enabling transseptal delivery in patients with moderate to severe or severe symptomatic mitral regurgitation at high surgical risk. Candidacy was determined by heart teams, with approval by a central screening committee. Echocardiographic data were evaluated by an independent core laboratory. Clinical events were adjudicated by a clinical events committee.
Results
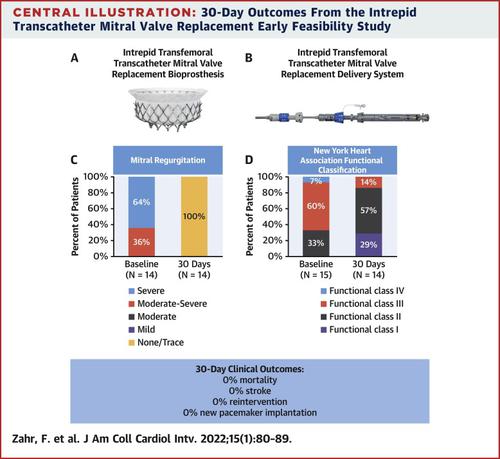
Fifteen patients were enrolled at 6 sites from February 2020 to May 2021. The median age was 80 years, and median Society of Thoracic Surgeons Predicted Risk of Mortality was 4.7%; 87% of patients were men, and 53% had undergone prior sternotomy. Fourteen implants were successful. One patient was converted to surgery during the index procedure. Patients stayed a median of 5 days postprocedure. There were 6 access site bleeds (40%) and 11 iatrogenic atrial septal defect closures (73%). At 30 days, there were no deaths, strokes, or reinterventions. All patients undergoing implantation had trace or no valvular or paravalvular mitral regurgitation, and the mean gradient was 4.7 mm Hg (IQR: 3.0-6.7 mm Hg).
Conclusions
Thirty-day results from the Intrepid transfemoral TMVR early feasibility study demonstrate excellent valve function and no mortality or stroke. Additional patients and longer follow-up are needed to confirm these findings. ([The Early Feasibility Study of the Intrepid™ TMVR Transseptal System]; NCT02322840)
中文翻译:

经股动脉经中隔经导管二尖瓣置换术后 30 天结果
目标
本研究的目的是评估经导管二尖瓣置换术 (TMVR) 与经股动脉通路在高手术风险患者中的结果。
背景
禁止手术风险可能会阻止某些患者进行二尖瓣置换手术。研究中的 Intrepid TMVR 系统之前已使用经心尖通路进行评估,以提供自膨胀生物瓣膜。
方法
这项前瞻性、多中心、非随机的早期可行性研究评估了 Intrepid 瓣膜的安全性和性能,该研究使用经股动脉通路在手术风险高的中度至重度或重度症状性二尖瓣关闭不全患者中实现经间隔分娩。候选资格由心脏团队确定,并经中央筛选委员会批准。超声心动图数据由独立的核心实验室评估。临床事件由临床事件委员会裁定。
结果
从 2020 年 2 月到 2021 年 5 月,在 6 个地点入组了 15 名患者。中位年龄为 80 岁,胸外科医师协会预测的中位死亡风险为 4.7%;87% 的患者为男性,53% 的患者曾接受过胸骨切开术。十四次植入成功。一名患者在索引过程中转为手术。患者在术后平均停留 5 天。有 6 例进入部位出血 (40%) 和 11 例医源性房间隔缺损闭合 (73%)。在 30 天时,没有死亡、中风或再次干预。所有接受植入的患者都有微量或无瓣膜或瓣周二尖瓣关闭不全,平均梯度为 4.7 mm Hg(IQR:3.0-6.7 mm Hg)。
结论
Intrepid transfemoral transfemoral TMVR 早期可行性研究的 30 天结果表明,瓣膜功能极佳,没有死亡或中风。需要更多的患者和更长的随访时间来证实这些发现。([Intrepid™ TMVR 经中隔系统的早期可行性研究];NCT02322840)











































 京公网安备 11010802027423号
京公网安备 11010802027423号