当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transketolase Catalyzed Synthesis of N-Aryl Hydroxamic Acids
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-04 , DOI: 10.1002/adsc.202101100 Inés Fúster-Fernández 1 , Laurence Hecquet 2 , Wolf-Dieter Fessner 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-04 , DOI: 10.1002/adsc.202101100 Inés Fúster-Fernández 1 , Laurence Hecquet 2 , Wolf-Dieter Fessner 1
Affiliation
|
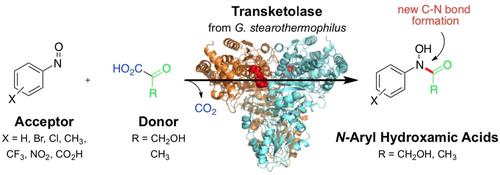
Hydroxamic acids are metal-chelating compounds that show important biological activity including anti-tumor effects. We have recently engineered the transketolase from Geobacillus stearothermopilus (TKgst) to convert benzaldehyde as a non-natural acceptor substrate. Realizing the structural and electronic similarity to nitrosobenzene, we studied the TK-catalyzed conversion of nitrosoarenes to yield N-arylated hydroxamic acids. Here we demonstrate that wild-type and variants of this versatile TKgst enzyme indeed induce the rapid biocatalytic conversion of variously p-, m- and o-substituted nitrosoarenes to produce a variety of corresponding N-aryl hydroxamic acids via creation of a carbon-nitrogen instead of a carbon-carbon bond. Further structural modifications can be introduced by varying the donor component, such as hydroxypyruvate or pyruvate.
中文翻译:

转酮酶催化合成N-芳基羟肟酸
异羟肟酸是一种金属螯合化合物,具有重要的生物活性,包括抗肿瘤作用。我们最近设计了来自Geobacillus stearothermopilus (TK gst ) 的转酮醇酶,以将苯甲醛转化为非天然受体底物。认识到与亚硝基苯的结构和电子相似性,我们研究了 TK 催化的亚硝基芳烃转化为N-芳基化异羟肟酸。在这里,我们证明了这种多功能 TK gst酶的野生型和变体确实诱导各种p-、m-和o-取代的亚硝基芳烃的快速生物催化转化,以产生各种相应的N-芳基异羟肟酸通过产生碳-氮而不是碳-碳键。通过改变供体组分,例如羟基丙酮酸或丙酮酸,可以引入进一步的结构修饰。
更新日期:2021-11-04
中文翻译:

转酮酶催化合成N-芳基羟肟酸
异羟肟酸是一种金属螯合化合物,具有重要的生物活性,包括抗肿瘤作用。我们最近设计了来自Geobacillus stearothermopilus (TK gst ) 的转酮醇酶,以将苯甲醛转化为非天然受体底物。认识到与亚硝基苯的结构和电子相似性,我们研究了 TK 催化的亚硝基芳烃转化为N-芳基化异羟肟酸。在这里,我们证明了这种多功能 TK gst酶的野生型和变体确实诱导各种p-、m-和o-取代的亚硝基芳烃的快速生物催化转化,以产生各种相应的N-芳基异羟肟酸通过产生碳-氮而不是碳-碳键。通过改变供体组分,例如羟基丙酮酸或丙酮酸,可以引入进一步的结构修饰。



























 京公网安备 11010802027423号
京公网安备 11010802027423号