当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile construction of peptidomimetics by sequential C–S/C–N bond activation of Ugi-adducts
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-30 , DOI: 10.1039/d1qo01438b Chao Liu 1 , Liangliang Song 2 , Vsevolod A. Peshkov 3, 4 , Erik V. Van der Eycken 1, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-30 , DOI: 10.1039/d1qo01438b Chao Liu 1 , Liangliang Song 2 , Vsevolod A. Peshkov 3, 4 , Erik V. Van der Eycken 1, 5
Affiliation
|
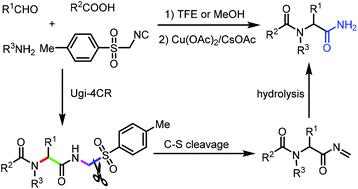
A novel selectively sequential C–S/C–N bond activation is presented. Through the combination of an Ugi-4CR and sequential C–S/C–N bond cleavage, diverse peptidomimetics containing a primary amide are prepared in a rapid, highly efficient and step-economical manner. This approach exhibits high yield, excellent chemoselectivity and functional group tolerance. Compounds derived from the pharmaceuticals febuxostat, probenecid and memantine as well as β-amino acid are prepared. This method provides a new direction for the synthesis of peptidomimetics.
中文翻译:

通过 Ugi 加合物的连续 C-S/C-N 键激活轻松构建肽模拟物
提出了一种新颖的选择性顺序 C-S/C-N 键活化。通过 Ugi-4CR 和连续 C-S/C-N 键断裂的组合,以快速、高效和步骤经济的方式制备了包含伯酰胺的多种肽模拟物。这种方法表现出高产率、优异的化学选择性和官能团耐受性。制备了衍生自药物非布司他、丙磺舒和美金刚以及β-氨基酸的化合物。该方法为拟肽的合成提供了新的方向。
更新日期:2021-11-05
中文翻译:

通过 Ugi 加合物的连续 C-S/C-N 键激活轻松构建肽模拟物
提出了一种新颖的选择性顺序 C-S/C-N 键活化。通过 Ugi-4CR 和连续 C-S/C-N 键断裂的组合,以快速、高效和步骤经济的方式制备了包含伯酰胺的多种肽模拟物。这种方法表现出高产率、优异的化学选择性和官能团耐受性。制备了衍生自药物非布司他、丙磺舒和美金刚以及β-氨基酸的化合物。该方法为拟肽的合成提供了新的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号