当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereocontrolled Synthesis of Enantiopure cis-Fused Octahydroisoindolones via Chiral Oxazoloisoindolone Lactams
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acs.joc.1c01757 Alberto Marbán-González 1 , Gaspar Maravilla-Moreno 1 , Josué Vazquez-Chavez 2 , Marcos Hernández-Rodríguez 2 , Rodrigo Said Razo-Hernández 3 , Mario Ordóñez 1 , José Luis Viveros-Ceballos 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acs.joc.1c01757 Alberto Marbán-González 1 , Gaspar Maravilla-Moreno 1 , Josué Vazquez-Chavez 2 , Marcos Hernández-Rodríguez 2 , Rodrigo Said Razo-Hernández 3 , Mario Ordóñez 1 , José Luis Viveros-Ceballos 1
Affiliation
|
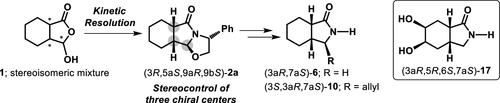
Kinetically controlled cyclocondensation of stereoisomeric and ring–chain tautomeric mixture of (±)-hydroxylactone 1 and 0.5 equiv of (R)-phenylglycinol provided tricyclic oxazoloisoindolone lactam (3R,5aS,9aR,9bS)-2a, a versatile intermediate for further stereocontrolled transformations to access enantiopure cis-fused octahydroisoindolones. An extension of this methodology was successfully applied to the synthesis of the 5,6-dihydroxy derivative (3aR,5R,6S,7aS)-17.
中文翻译:

手性恶唑并异吲哚酮内酰胺立体控制合成对映体顺式稠合八氢异吲哚酮
(±)-羟基内酯1和 0.5 equiv 的 ( R )-苯基甘氨醇的立体异构体和环链互变异构体混合物的动力学控制环缩合提供三环恶唑并异吲哚酮内酰胺 (3 R ,5a S ,9a R ,9b S ) -2a,一种多功能中间体用于进一步立体控制转化以获得对映体纯顺式稠合八氢异吲哚酮。该方法的扩展成功地应用于 5,6-二羟基衍生物 (3a R ,5 R ,6 S ,7a S )- 17的合成。
更新日期:2021-12-03
中文翻译:

手性恶唑并异吲哚酮内酰胺立体控制合成对映体顺式稠合八氢异吲哚酮
(±)-羟基内酯1和 0.5 equiv 的 ( R )-苯基甘氨醇的立体异构体和环链互变异构体混合物的动力学控制环缩合提供三环恶唑并异吲哚酮内酰胺 (3 R ,5a S ,9a R ,9b S ) -2a,一种多功能中间体用于进一步立体控制转化以获得对映体纯顺式稠合八氢异吲哚酮。该方法的扩展成功地应用于 5,6-二羟基衍生物 (3a R ,5 R ,6 S ,7a S )- 17的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号