当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Oxacycles via Ruthenium-Catalyzed Atom-Economic Coupling of Propargyl Alcohols and Michael Acceptors
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-04 , DOI: 10.1021/acs.joc.1c01758 Nabakumar Bera 1 , Shantanu Samanta 1 , Debayan Sarkar 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-04 , DOI: 10.1021/acs.joc.1c01758 Nabakumar Bera 1 , Shantanu Samanta 1 , Debayan Sarkar 1
Affiliation
|
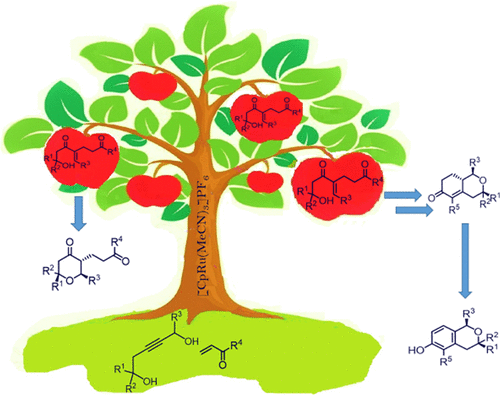
Synthesis of β-hydroxyenones and its application toward development of tetrahydro-4H-pyran-4-one in an atom-economic fashion is limited. This manuscript describes a ruthenium-catalyzed atom-economic coupling of pent-2-yne-1,5-diols and Michael acceptors as an efficient route for the synthesis of β-hydroxyenones with excellent yields and high regioselectivity. The β-hydroxyenones further undergo a 6-endo trig cyclization under acid-catalyzed conditions to deliver the tetrahydro-4H-pyran-4-ones with high diastereoselectivity. An intramolecular aldol condensation under mild basic conditions and palladium-catalyzed oxidative aromatization was developed for the synthesis of hexahydro-6H-isochromen-6-ones and isochromanols, respectively, from highly substituted tetrahydro-4H-pyran-4-ones with excellent yield and diastereoselectivity. Overall, this work demonstrates the synthetic potential toward the synthesis of oxacycles like tetrahydro-4H-pyran-4-ones, hexahydro-6H-isochromen-6-ones, and isochromanols via an atom-economic catalysis.
中文翻译:

钌催化炔丙醇和迈克尔受体的原子经济偶联立体选择性合成氧杂环
β-羟基烯酮的合成及其在以原子经济方式开发四氢-4 H-吡喃-4-酮方面的应用受到限制。该手稿描述了钌催化的 pent-2-yne-1,5-二醇和迈克尔受体的原子经济耦合,作为合成具有优异收率和高区域选择性的 β-羟基烯酮的有效途径。β-羟基烯酮在酸催化条件下进一步经历 6-内三环化以提供具有高非对映选择性的四氢-4 H-吡喃-4-酮。开发了温和碱性条件下的分子内羟醛缩合和钯催化的氧化芳构化合成hexahydro-6 H-isochromen-6-ones 和 isochromanols,分别来自高度取代的四氢-4 H -pyran-4-ones,具有优异的产率和非对映选择性。总体而言,这项工作展示了通过原子经济催化合成氧杂环化合物(如四氢-4 H-吡喃-4-酮、六氢-6 H-异色烯-6-酮和异色甘醇)的合成潜力。
更新日期:2021-12-03
中文翻译:

钌催化炔丙醇和迈克尔受体的原子经济偶联立体选择性合成氧杂环
β-羟基烯酮的合成及其在以原子经济方式开发四氢-4 H-吡喃-4-酮方面的应用受到限制。该手稿描述了钌催化的 pent-2-yne-1,5-二醇和迈克尔受体的原子经济耦合,作为合成具有优异收率和高区域选择性的 β-羟基烯酮的有效途径。β-羟基烯酮在酸催化条件下进一步经历 6-内三环化以提供具有高非对映选择性的四氢-4 H-吡喃-4-酮。开发了温和碱性条件下的分子内羟醛缩合和钯催化的氧化芳构化合成hexahydro-6 H-isochromen-6-ones 和 isochromanols,分别来自高度取代的四氢-4 H -pyran-4-ones,具有优异的产率和非对映选择性。总体而言,这项工作展示了通过原子经济催化合成氧杂环化合物(如四氢-4 H-吡喃-4-酮、六氢-6 H-异色烯-6-酮和异色甘醇)的合成潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号