当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multidimensional Free Energy and Accelerated Quantum Library Methods Provide a Gateway to Glycoenzyme Conformational, Electronic, and Reaction Mechanisms
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-11-02 , DOI: 10.1021/acs.accounts.1c00477 Kevin J Naidoo 1, 2 , Tomás Bruce-Chwatt 1 , Tharindu Senapathi 1 , Malcolm Hillebrand 1, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-11-02 , DOI: 10.1021/acs.accounts.1c00477 Kevin J Naidoo 1, 2 , Tomás Bruce-Chwatt 1 , Tharindu Senapathi 1 , Malcolm Hillebrand 1, 3
Affiliation
|
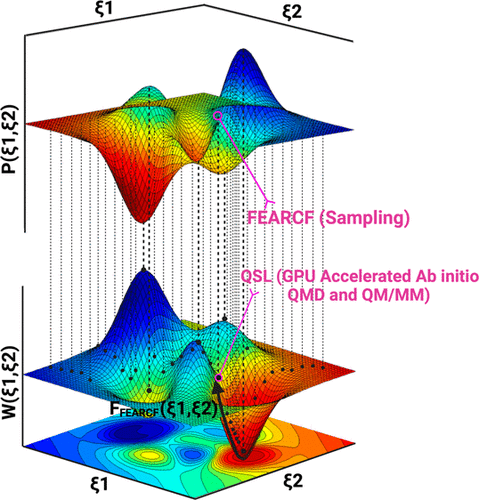
Enzyme reactions are complex to simulate accurately, and none more so than glycoenzymes (glycosyltransferase and glycosidases). A rigorous sampling of the protein frame and the conformationally plural carbohydrate substrate coupled with an unbiased treatment of the electron dynamics is needed to discover the true reaction landscapes. Here, we demonstrate the effectiveness of two computational methods ported in libraries that we have developed. The first is a flat histogram free energy method called FEARCF capable of multidimensional sampling and rapidly converging to a complete coverage of phase space. The second, the Quantum Supercharger Library (QSL), is a method that accelerates the computation of the ab initio electronic wave function as well as the integral derivatives on graphical processing units (GPUs). These QSL accelerated computations form the core components needed for direct quantum dynamics and QM/MM dynamics when coupled with legacy codes such as GAMESS and NWCHEM, making state of the art hyper-parallel electronic computations in chemistry and chemical biology possible. The combination of QSL (acceleration of ab initio QM computation) and FEARCF (multidimensional hyper-parallel reaction dynamics) makes the simulation of ab initio QM/MM reaction dynamics of enzyme catalysis feasible. Enzymes that process carbohydrates pose an added challenge as their pyranose ring substrates span multidimensional conformational space whose sampling is an intimate function of the catalytic mechanism. Here, we use the pairing of FEARCF and QSL to simulate the catalytic effect of the glycoenzyme β-N-acetylglucosamine transferase (OGT). The reaction mechanism is discovered from a variable three bond reaction surface using SCCDFTB. The role of the OGT in distorting the pyranose ring of β-N-acetylglucosamine (GlcNAc) away from the equilibrium 4C1 chair conformation toward the E3 envelope needed for the transition state is discovered from its pucker free energy hypersurfaces (or free energy volume, FEV). A complete GlcNAc ring pucker HF 6-31g FEV is constructed from ab initio QM dynamics in vacuum and ab initio QM/MM dynamics in the OGT catalytic domain. The OGT is shown to clearly lower the pathway toward the transition state E3 ring conformer as well as stabilize it by 1.63 kcal/mol. Illustrated here is the use of QSL accelerated ab initio QM/MM dynamics that thoroughly explores carbohydrate catalyzed reactions through a FEARCF multidimensional sampling of the interdependence between reaction and conformational space. This demonstrates how experimentally inaccessible molecular and electronic mechanisms that underpin enzyme catalysis can be discovered by directly modeling the dynamics of these complex reactions.
中文翻译:

多维自由能和加速量子库方法为糖酶构象、电子和反应机制提供了途径
酶反应非常复杂,难以准确模拟,尤其是糖酶(糖基转移酶和糖苷酶)。需要对蛋白质框架和构象多元碳水化合物底物进行严格采样,并结合电子动力学的公正处理,以发现真正的反应景观。在这里,我们展示了我们开发的库中移植的两种计算方法的有效性。第一种是称为 FEARCF 的平面直方图自由能方法,能够进行多维采样并快速收敛到完全覆盖相空间。第二个是量子增压器库 (QSL),它是一种加速从头算电子波函数以及图形处理单元 (GPU) 上积分导数计算的方法。当与 GAMESS 和 NWCHEM 等传统代码结合使用时,这些 QSL 加速计算构成了直接量子动力学和 QM/MM 动力学所需的核心组件,使化学和化学生物学中最先进的超并行电子计算成为可能。 QSL(加速从头计算 QM 计算)和 FEARCF(多维超并行反应动力学)的结合使得酶催化从头计算 QM/MM 反应动力学的模拟变得可行。处理碳水化合物的酶提出了额外的挑战,因为它们的吡喃糖环底物跨越多维构象空间,其采样是催化机制的密切功能。在这里,我们使用 FEARCF 和 QSL 配对来模拟糖酶 β- N-乙酰氨基葡萄糖转移酶 (OGT) 的催化作用。使用 SCCDFTB 从可变三键反应表面发现了反应机理。 OGT 在扭曲 β- N-乙酰氨基葡萄糖 (GlcNAc) 的吡喃糖环中的作用,从平衡4 C 1椅构象转向过渡态所需的 E 3包络,是从其褶皱自由能超曲面(或自由能)中发现的体积、FEV)。完整的 GlcNAc 环褶皱 HF 6-31g FEV 由真空中的从头开始 QM 动力学和 OGT 催化域中的从头开始 QM/MM 动力学构建。 OGT 显示出明显降低了过渡态 E 3环构象异构体的路径,并将其稳定了 1.63 kcal/mol。这里说明的是使用 QSL 加速从头开始 QM/MM 动力学,通过反应和构象空间之间相互依赖性的 FEARCF 多维采样彻底探索碳水化合物催化反应。这证明了如何通过直接模拟这些复杂反应的动力学来发现实验上无法实现的支持酶催化的分子和电子机制。
更新日期:2021-11-16
中文翻译:

多维自由能和加速量子库方法为糖酶构象、电子和反应机制提供了途径
酶反应非常复杂,难以准确模拟,尤其是糖酶(糖基转移酶和糖苷酶)。需要对蛋白质框架和构象多元碳水化合物底物进行严格采样,并结合电子动力学的公正处理,以发现真正的反应景观。在这里,我们展示了我们开发的库中移植的两种计算方法的有效性。第一种是称为 FEARCF 的平面直方图自由能方法,能够进行多维采样并快速收敛到完全覆盖相空间。第二个是量子增压器库 (QSL),它是一种加速从头算电子波函数以及图形处理单元 (GPU) 上积分导数计算的方法。当与 GAMESS 和 NWCHEM 等传统代码结合使用时,这些 QSL 加速计算构成了直接量子动力学和 QM/MM 动力学所需的核心组件,使化学和化学生物学中最先进的超并行电子计算成为可能。 QSL(加速从头计算 QM 计算)和 FEARCF(多维超并行反应动力学)的结合使得酶催化从头计算 QM/MM 反应动力学的模拟变得可行。处理碳水化合物的酶提出了额外的挑战,因为它们的吡喃糖环底物跨越多维构象空间,其采样是催化机制的密切功能。在这里,我们使用 FEARCF 和 QSL 配对来模拟糖酶 β- N-乙酰氨基葡萄糖转移酶 (OGT) 的催化作用。使用 SCCDFTB 从可变三键反应表面发现了反应机理。 OGT 在扭曲 β- N-乙酰氨基葡萄糖 (GlcNAc) 的吡喃糖环中的作用,从平衡4 C 1椅构象转向过渡态所需的 E 3包络,是从其褶皱自由能超曲面(或自由能)中发现的体积、FEV)。完整的 GlcNAc 环褶皱 HF 6-31g FEV 由真空中的从头开始 QM 动力学和 OGT 催化域中的从头开始 QM/MM 动力学构建。 OGT 显示出明显降低了过渡态 E 3环构象异构体的路径,并将其稳定了 1.63 kcal/mol。这里说明的是使用 QSL 加速从头开始 QM/MM 动力学,通过反应和构象空间之间相互依赖性的 FEARCF 多维采样彻底探索碳水化合物催化反应。这证明了如何通过直接模拟这些复杂反应的动力学来发现实验上无法实现的支持酶催化的分子和电子机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号