当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystallization, structural characterization and kinetic analysis of a GH26 β-mannanase from Klebsiella oxytoca KUB-CW2-3
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2059798321009992 Nawapan Pongsapipatana 1 , Ratana Charoenwattanasatien 2 , Nuttawan Pramanpol 3 , Thu Ha Nguyen 4 , Dietmar Haltrich 4 , Sunee Nitisinprasert 1 , Suttipun Keawsompong 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2059798321009992 Nawapan Pongsapipatana 1 , Ratana Charoenwattanasatien 2 , Nuttawan Pramanpol 3 , Thu Ha Nguyen 4 , Dietmar Haltrich 4 , Sunee Nitisinprasert 1 , Suttipun Keawsompong 1
Affiliation
|
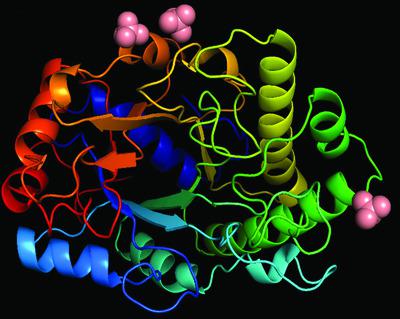
β-Mannanase (EC 3.2.1.78) is an enzyme that cleaves within the backbone of mannan-based polysaccharides at β-1,4-linked d-mannose residues, resulting in the formation of mannooligosaccharides (MOS), which are potential prebiotics. The GH26 β-mannanase KMAN from Klebsiella oxytoca KUB-CW2-3 shares 49–72% amino-acid sequence similarity with β-mannanases from other sources. The crystal structure of KMAN at a resolution of 2.57 Å revealed an open cleft-shaped active site. The enzyme structure is based on a (β/α)8-barrel architecture, which is a typical characteristic of clan A glycoside hydrolase enzymes. The putative catalytic residues Glu183 and Glu282 are located on the loop connected to β-strand 4 and at the end of β-strand 7, respectively. KMAN digests linear MOS with a degree of polymerization (DP) of between 4 and 6, with high catalytic efficiency (kcat/Km) towards DP6 (2571.26 min−1 mM−1). The predominant end products from the hydrolysis of locust bean gum, konjac glucomannan and linear MOS are mannobiose and mannotriose. It was observed that KMAN requires at least four binding sites for the binding of substrate molecules and hydrolysis. Molecular docking of mannotriose and galactosyl-mannotetraose to KMAN confirmed its mode of action, which prefers linear substrates to branched substrates.
中文翻译:

产酸克雷伯菌 KUB-CW2-3 GH26 β-甘露聚糖酶的结晶、结构表征和动力学分析
β-甘露聚糖酶 (EC 3.2.1.78) 是一种酶,它在甘露聚糖基多糖骨架中的 β-1,4 连接的d-甘露糖残基处切割,从而形成甘露寡糖 (MOS),这是潜在的益生元。产酸克雷伯菌KUB-CW2-3的 GH26 β-甘露聚糖酶 KMAN与其他来源的 β-甘露聚糖酶具有 49-72% 的氨基酸序列相似性。分辨率为 2.57 Å 的 KMAN 晶体结构揭示了一个开放的裂隙状活性位点。酶结构基于 (β/α) 8-桶形结构,这是A族糖苷水解酶的典型特征。推定的催化残基 Glu183 和 Glu282 分别位于与 β-链 4 连接的环上和 β-链 7 的末端。KMAN 消化聚合度 (DP) 介于 4 和 6 之间的线性 MOS,对 DP6 具有高催化效率 ( k cat / K m ) (2571.26 min -1 m M -1)。刺槐豆胶、魔芋葡甘聚糖和线性MOS水解的主要最终产物是甘露二糖和甘露三糖。据观察,KMAN 需要至少四个结合位点来结合底物分子和水解。甘露三糖和半乳糖基甘露四糖与 KMAN 的分子对接证实了其作用方式,它更喜欢线性底物而不是支链底物。
更新日期:2021-11-03
中文翻译:

产酸克雷伯菌 KUB-CW2-3 GH26 β-甘露聚糖酶的结晶、结构表征和动力学分析
β-甘露聚糖酶 (EC 3.2.1.78) 是一种酶,它在甘露聚糖基多糖骨架中的 β-1,4 连接的d-甘露糖残基处切割,从而形成甘露寡糖 (MOS),这是潜在的益生元。产酸克雷伯菌KUB-CW2-3的 GH26 β-甘露聚糖酶 KMAN与其他来源的 β-甘露聚糖酶具有 49-72% 的氨基酸序列相似性。分辨率为 2.57 Å 的 KMAN 晶体结构揭示了一个开放的裂隙状活性位点。酶结构基于 (β/α) 8-桶形结构,这是A族糖苷水解酶的典型特征。推定的催化残基 Glu183 和 Glu282 分别位于与 β-链 4 连接的环上和 β-链 7 的末端。KMAN 消化聚合度 (DP) 介于 4 和 6 之间的线性 MOS,对 DP6 具有高催化效率 ( k cat / K m ) (2571.26 min -1 m M -1)。刺槐豆胶、魔芋葡甘聚糖和线性MOS水解的主要最终产物是甘露二糖和甘露三糖。据观察,KMAN 需要至少四个结合位点来结合底物分子和水解。甘露三糖和半乳糖基甘露四糖与 KMAN 的分子对接证实了其作用方式,它更喜欢线性底物而不是支链底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号