JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2021-11-01 , DOI: 10.1016/j.jcin.2021.07.045 Tetsuya Matoba 1 , Satoshi Yasuda 2 , Koichi Kaikita 3 , Masaharu Akao 4 , Junya Ako 5 , Masato Nakamura 6 , Katsumi Miyauchi 7 , Nobuhisa Hagiwara 8 , Kazuo Kimura 9 , Atsushi Hirayama 10 , Kunihiko Matsui 11 , Hisao Ogawa 12 ,
|
Objectives
The aim of this AFIRE (Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease) trial subgroup analysis was to examine rivaroxaban monotherapy benefits and their relation to the time between stenting and enrollment among patients after coronary stenting.
Background
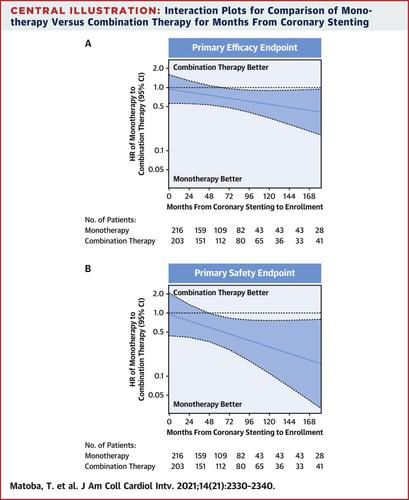
Of 2,215 patients with atrial fibrillation and stable coronary artery disease in the AFIRE trial, rivaroxaban monotherapy was noninferior to rivaroxaban plus antiplatelet therapy (combination therapy) in terms of efficacy and superior for safety endpoints. However, thrombotic risk after antiplatelet therapy cessation remained a concern among 1,444 patients who had undergone coronary stenting >1 year before enrollment.
Methods
The benefits of rivaroxaban monotherapy in coronary stenting subgroups were assessed for efficacy (a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, or death of any cause), safety (major bleeding defined according to International Society on Thrombosis and Haemostasis criteria), ischemic endpoints, net adverse clinical event, and time between stenting and enrollment.
Results
Efficacy and safety endpoints for monotherapy were superior to combination therapy, with HRs of 0.70 for efficacy (95% CI: 0.50-0.98; P = 0.036) and 0.55 for safety (95% CI: 0.33-0.92; P = 0.019). For ischemic endpoints, the HR was 0.82 (95% CI: 0.58-1.15; P = 0.240). The HR became smaller with longer time between stenting and enrollment (efficacy, P for interaction = 0.158; safety, P = 0.097).
Conclusions
In patients with atrial fibrillation after coronary stenting, the benefits of rivaroxaban monotherapy for efficacy and safety endpoints were consistent with those in the whole AFIRE trial population. The benefits became apparent with longer time between stenting and enrollment. (Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease Study [AFIRE]; UMIN000016612, NCT02642419)
中文翻译:

冠状动脉支架置入术后心房颤动患者的利伐沙班单药治疗
目标
这项 AFIRE(利伐沙班在稳定冠状动脉疾病患者中的心房颤动和缺血事件)试验亚组分析的目的是检查利伐沙班单药治疗的益处及其与冠状动脉支架置入后患者中支架置入和入组之间时间的关系。
背景
在 AFIRE 试验的 2,215 名房颤和稳定型冠状动脉疾病患者中,利伐沙班单药治疗在疗效方面不劣于利伐沙班加抗血小板治疗(联合治疗),并且在安全性终点方面优于利伐沙班。然而,在入组前 1 年以上接受过冠状动脉支架置入术的 1,444 名患者中,停止抗血小板治疗后的血栓形成风险仍然令人担忧。
方法
评估了利伐沙班单药治疗在冠状动脉支架亚组中的疗效(包括中风、全身性栓塞、心肌梗死、需要血运重建的不稳定型心绞痛或任何原因的死亡)、安全性(根据国际血栓和止血学会定义的大出血)标准)、缺血性终点、净不良临床事件以及支架植入和入组之间的时间。
结果
单药治疗的疗效和安全性终点优于联合治疗,疗效的 HR 为 0.70(95% CI:0.50-0.98;P = 0.036),安全性为 0.55(95% CI:0.33-0.92;P = 0.019)。对于缺血性终点,HR 为 0.82(95% CI:0.58-1.15;P = 0.240)。随着支架植入和入组之间时间的延长,HR 变得更小(疗效,P交互作用 = 0.158;安全性,P = 0.097)。
结论
在冠状动脉支架置入术后发生心房颤动的患者中,利伐沙班单药治疗在疗效和安全性终点方面的益处与整个 AFIRE 试验人群的益处一致。随着支架植入和入组之间的时间延长,这些好处变得明显。(利伐沙班在稳定型冠状动脉疾病患者中的心房颤动和缺血事件研究 [AFIRE];UMIN000016612,NCT02642419)









































 京公网安备 11010802027423号
京公网安备 11010802027423号