当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination, Solute–Solvent Interactions, and Model Correlation of Sorafenib in Twelve Pure Organic Solvents between T = 273.15 and 313.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-11-01 , DOI: 10.1021/acs.jced.1c00279 Yüfang Wu 1 , Qingzhi Guo 1 , Jianhua Bai 1 , Chunyan Guo 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-11-01 , DOI: 10.1021/acs.jced.1c00279 Yüfang Wu 1 , Qingzhi Guo 1 , Jianhua Bai 1 , Chunyan Guo 1
Affiliation
|
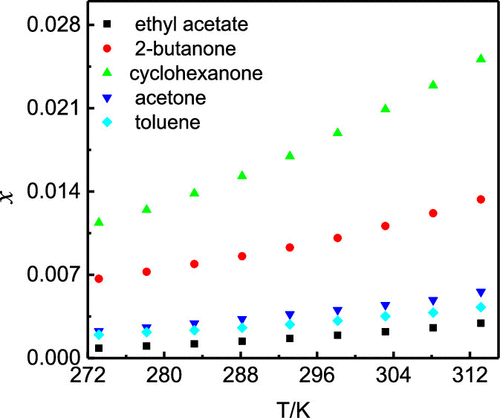
The isothermal saturation method was applied to study the solubility of sorafenib free base in 12 pure solvents in the temperature range from 273.15 to 313.15 K under atmospheric pressure. The solubility in selected solvents increased with the increasing temperature. The maximum mole solubility was in cyclohexanone (2.512 × 10–2) at 313.15 K, followed by in 2-butanone (1.334 × 10–2), acetone (5.577 × 10–3), toluene (4.282 × 10–3), ethyl acetate (2.930 × 10–3), n-propanol (2.067 × 10–3), 1-butanol (1.816 × 10–3), ethanol (1.554 × 10–3), isopropanol (1.385 × 10–3), isobutanol (1.256 × 10–3), methanol (0.848 × 10–3), and acetonitrile (0.678 × 10–3). The quantitative solubility data of sorafenib free base in some pure and mixed organic solvents have been reported in the literature. However, the results were not satisfactory. There is an increase of 12.06-fold between cyclohexanone and n-propanol at 313.15 K. Hence, for sorafenib free base, the solubility can be improved not only in the form of salt, but also by the selection of a good solvent. In addition, the results obtained in 12 pure solvents were correlated with the modified Apelblat equation and the λh equation. The maximum of relative average deviation was 1.62% and obtained using the λh equation, which indicated that two equations could give satisfactory results in selected pure solvents.
中文翻译:

索拉非尼在 T = 273.15 和 313.15 K 之间的十二种纯有机溶剂中的溶解度测定、溶质-溶剂相互作用和模型相关性
应用等温饱和法研究了索拉非尼游离碱在 273.15 至 313.15 K 的温度范围内在大气压下在 12 种纯溶剂中的溶解度。在选定溶剂中的溶解度随着温度的升高而增加。最大摩尔溶解度在 313.15 K时在环己酮 (2.512 × 10 –2 ) 中,其次是在 2-丁酮 (1.334 × 10 –2 )、丙酮 (5.577 × 10 –3 )、甲苯 (4.282 × 10 –3 )、乙酸乙酯 (2.930 × 10 –3 )、正丙醇(2.067 × 10 –3 )、1-丁醇 (1.816 × 10 –3 )、乙醇 (1.554 × 10 –3 )、异丙醇 (1.385 × 10 –3 ))、异丁醇 (1.256 × 10 –3 )、甲醇 (0.848 × 10 –3 ) 和乙腈 (0.678 × 10 –3 )。索拉非尼游离碱在一些纯有机溶剂和混合有机溶剂中的定量溶解度数据已有文献报道。然而,结果并不令人满意。在313.15 K时,环己酮和正丙醇之间增加了12.06倍。因此,对于索拉非尼游离碱,不仅可以以盐的形式,而且可以通过选择良好的溶剂来提高溶解度。此外,在 12 种纯溶剂中获得的结果与修改后的 Apelblat 方程和 λ h方程相关联。相对平均偏差的最大值为 1.62%,使用 λ h获得 方程,这表明两个方程可以在选定的纯溶剂中得到令人满意的结果。
更新日期:2021-12-09
中文翻译:

索拉非尼在 T = 273.15 和 313.15 K 之间的十二种纯有机溶剂中的溶解度测定、溶质-溶剂相互作用和模型相关性
应用等温饱和法研究了索拉非尼游离碱在 273.15 至 313.15 K 的温度范围内在大气压下在 12 种纯溶剂中的溶解度。在选定溶剂中的溶解度随着温度的升高而增加。最大摩尔溶解度在 313.15 K时在环己酮 (2.512 × 10 –2 ) 中,其次是在 2-丁酮 (1.334 × 10 –2 )、丙酮 (5.577 × 10 –3 )、甲苯 (4.282 × 10 –3 )、乙酸乙酯 (2.930 × 10 –3 )、正丙醇(2.067 × 10 –3 )、1-丁醇 (1.816 × 10 –3 )、乙醇 (1.554 × 10 –3 )、异丙醇 (1.385 × 10 –3 ))、异丁醇 (1.256 × 10 –3 )、甲醇 (0.848 × 10 –3 ) 和乙腈 (0.678 × 10 –3 )。索拉非尼游离碱在一些纯有机溶剂和混合有机溶剂中的定量溶解度数据已有文献报道。然而,结果并不令人满意。在313.15 K时,环己酮和正丙醇之间增加了12.06倍。因此,对于索拉非尼游离碱,不仅可以以盐的形式,而且可以通过选择良好的溶剂来提高溶解度。此外,在 12 种纯溶剂中获得的结果与修改后的 Apelblat 方程和 λ h方程相关联。相对平均偏差的最大值为 1.62%,使用 λ h获得 方程,这表明两个方程可以在选定的纯溶剂中得到令人满意的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号