当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A photoredox/nickel dual-catalytic strategy for benzylic C–H alkoxylation
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-26 , DOI: 10.1039/d1qo01421h Min Dong 1 , Yuqi Jia 1 , Wei Zhou 1 , Jinlai Gao 1 , Xiaoqing Lv 1 , Fan Luo 2 , Yongqiang Zhang 2 , Shihui Liu 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-26 , DOI: 10.1039/d1qo01421h Min Dong 1 , Yuqi Jia 1 , Wei Zhou 1 , Jinlai Gao 1 , Xiaoqing Lv 1 , Fan Luo 2 , Yongqiang Zhang 2 , Shihui Liu 1
Affiliation
|
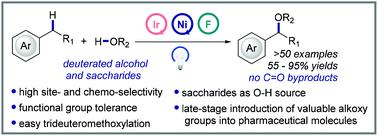
Benzylic C–H alkoxylation via C–H/O–H cross-coupling represents an attractive strategy to access highly functionalized benzylic ethers but remains challenging due to the difficulty in improving the efficiency and selectivity. Herein we wish to report a highly efficient photoredox/nickel dual-catalytic strategy for the site-selective and chemoselective benzylic C–H alkoxylation using readily available Selectfluor as the oxidant. The protocol features broad substrate scope and excellent functional group compatibility and allows the transformation of various of alcohols to pharmaceutically relevant benzylic ethers in moderate to excellent yields under mild reaction conditions. CD3OD was well tolerated, providing a useful synthetic tool for the synthesis of valuable deuterated pharmaceutical molecules. This method is also highlighted by the late-stage introduction of valuable alkoxy groups into pharmaceutical molecules and the structure modification of saccharides.
中文翻译:

用于苄基C-H烷氧基化的光氧化还原/镍双催化策略
通过C-H/O-H 交叉偶联进行的苄基C-H 烷氧基化代表了一种获得高度官能化苄基醚的有吸引力的策略,但由于难以提高效率和选择性,因此仍然具有挑战性。在此,我们希望报告一种高效的光氧化还原/镍双催化策略,用于使用现成的 Selectfluor 作为氧化剂进行位点选择性和化学选择性苄基 C-H 烷氧基化。该协议具有广泛的底物范围和出色的官能团兼容性,并允许在温和的反应条件下以中等至优异的产率将各种醇转化为药学相关的苄醚。光盘3OD 具有良好的耐受性,为合成有价值的氘代药物分子提供了有用的合成工具。这种方法还通过后期将有价值的烷氧基引入药物分子和糖类的结构修饰而突出显示。
更新日期:2021-11-01
中文翻译:

用于苄基C-H烷氧基化的光氧化还原/镍双催化策略
通过C-H/O-H 交叉偶联进行的苄基C-H 烷氧基化代表了一种获得高度官能化苄基醚的有吸引力的策略,但由于难以提高效率和选择性,因此仍然具有挑战性。在此,我们希望报告一种高效的光氧化还原/镍双催化策略,用于使用现成的 Selectfluor 作为氧化剂进行位点选择性和化学选择性苄基 C-H 烷氧基化。该协议具有广泛的底物范围和出色的官能团兼容性,并允许在温和的反应条件下以中等至优异的产率将各种醇转化为药学相关的苄醚。光盘3OD 具有良好的耐受性,为合成有价值的氘代药物分子提供了有用的合成工具。这种方法还通过后期将有价值的烷氧基引入药物分子和糖类的结构修饰而突出显示。



























 京公网安备 11010802027423号
京公网安备 11010802027423号