Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2021-10-31 , DOI: 10.1016/j.apcatb.2021.120881 Chun He 1 , Yuhong Liao 1 , Cheng Chen 1 , Dehua Xia 1 , Yongyi Wang 1 , Shuanghong Tian 1 , Jingling Yang 2 , Dong Shu 3
|
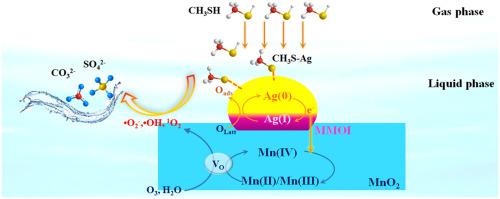
A novel process for S-VOCs degradation using wet scrubbing coupled with catalytic ozonation was developed for first time. We report redox-robust catalysts consisting of Ag(0)/Ag(I) species decorated rambutan-like MnO2 hollow microspheres (Ag/R-MnO2) with strong metal-metal oxide interaction (MMOI) exhibited superior catalytic ozonation performance for CH3SH elimination. The optimum Ag/R-MnO2 reached a significant improvement in CH3SH elimination of 96.9% conversion over pristine R-MnO2 under GHSV of 75,000 mL h−1 g−1, and O3 utilization rate of 92.3%. An outstanding stability of Ag/R-MnO2 under wet catalytic ozonation process was demonstrated, which outperformed that in gaseous system. CH3SH was captured by aqueous solution and preferentially chemisorbed on Ag, then deeply oxidized to the final products of SO42-/CO32- via catalytic ozonation by multivalent R-MnO2. The excellent performance can be ascribed to efficient electron replenishing interaction between Ag(0)/Ag(I) and multivalent R-MnO2, efficient O3 activation through oxygen vacancies-rich R-MnO2, and enhanced mass diffusion in wet scrubbing process.
中文翻译:

实现氧化还原稳定的 Ag/MnO2 催化剂,用于 S-VOCs 的高效湿法催化臭氧化:Ag(0)/Ag(I)-Mn 基氧化还原梭的促进作用
首次开发了一种使用湿法洗涤结合催化臭氧化降解 S-VOC 的新工艺。我们报告了由 Ag(0)/Ag(I) 物种装饰的红毛丹状 MnO 2空心微球 (Ag/R-MnO 2 )组成的氧化还原稳健催化剂,具有强金属-金属氧化物相互作用 (MMOI),表现出优异的催化臭氧化性能。 CH 3 SH消除。在75,000 mL h -1 g -1 的GHSV和92.3%的O 3利用率下,最佳的Ag/R-MnO 2在CH 3 SH消除方面达到了比原始R-MnO 2 96.9%的转化率的显着改进。Ag/R-MnO 2的出色稳定性展示了湿法催化臭氧化过程,其性能优于气态系统。CH 3 SH 被水溶液捕获并优先化学吸附在Ag 上,然后通过多价R-MnO 2催化臭氧化将其深度氧化为SO 4 2- /CO 3 2-最终产物。优异的性能可归因于 Ag(0)/Ag(I) 和多价 R-MnO 2之间有效的电子补充相互作用,通过富含氧空位的 R-MnO 2有效激活O 3,以及在湿法洗涤过程中增强的质量扩散.



























 京公网安备 11010802027423号
京公网安备 11010802027423号