Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-10-30 , DOI: 10.1016/j.jcat.2021.10.030 Guang-Jie Xia 1 , Yang-Gang Wang 1
|
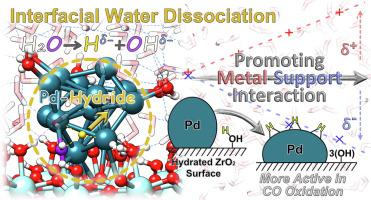
The supported metal catalysts are usually prepared and widely applied in aqueous phase. Here, by performing density functional theory calculations and ab initio molecular dynamics simulations with explicit solvent waters, the metal-support interaction of Pd@ZrO2 catalyst was found to be enhanced by water dissociation at the metal-support interface. This happens to an appreciable number of interfacial waters, generating ∼ 25% hydrides compared to Pd atoms. After the partially heterolytic water dissociation, the generated Hδ+ would undergo the proton coupled electron transfer (PCET) from Pd to form the hydride (Hδ-). The spatial separation of the generated H on Pd and remaining OH on ZrO2 results in the polarization of metal-support interface, which then flattens the shape of cluster. Moreover, the formed metal-hydride behaves as the new catalytic active phase for CO oxidation, making the catalyst highly active in water vapor.
中文翻译:

溶剂促进 Pd@ZrO2 催化剂的金属-载体相互作用和活性:在固液界面形成金属氢化物作为新的催化活性相
负载型金属催化剂通常在水相中制备并广泛应用。在这里,通过使用显式溶剂水进行密度泛函理论计算和从头分子动力学模拟,发现Pd@ZrO 2催化剂的金属-载体相互作用因金属-载体界面处的水离解而增强。这发生在相当多的界面水上,与 Pd 原子相比,产生了约 25% 的氢化物。在部分异裂水解离后,生成的 H δ+将经历来自 Pd 的质子耦合电子转移 (PCET) 以形成氢化物 (H δ- )。Pd 上生成的 H 和 ZrO 2上剩余的 OH 的空间分离导致金属-支撑界面的极化,然后使簇的形状变平。此外,形成的金属氢化物作为 CO 氧化的新催化活性相,使催化剂在水蒸气中具有高活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号