当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Functionalized 4,1-Benzothiazepines via a [4+3] Annulation between Aza-o-Quinone Methides and Pyridinium 1,4-Zwitterionic Thiolates
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/adsc.202101034 Chuan-Chuan Wang 1 , Xue-Hua Liu 1 , Xin-Lu Wang 2 , Hua-Peng Cui 3 , Zhi-Wei Ma 1 , Degang Ding 4 , Jun-Tao Liu 4 , Lei Meng 5 , Ya-Jing Chen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/adsc.202101034 Chuan-Chuan Wang 1 , Xue-Hua Liu 1 , Xin-Lu Wang 2 , Hua-Peng Cui 3 , Zhi-Wei Ma 1 , Degang Ding 4 , Jun-Tao Liu 4 , Lei Meng 5 , Ya-Jing Chen 1
Affiliation
|
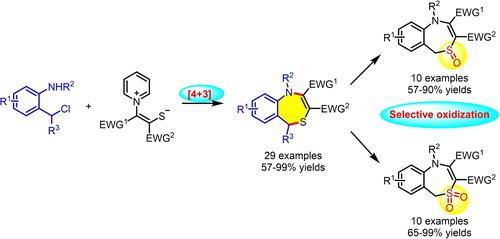
The [4+3] annulation of aza-o-quinone methides and pyridinium 1,4-zwitterionic thiolates has been developed for the one-step synthesis of functionalized 2,3-unsaturated 4,1-benzothiazepines under mild and metal-free conditions. The produced 4,1-benzothiazepines can easily be converted into biologically interesting sulfoxides and sulfones via selective oxidization with m-CPBA.
中文翻译:

通过氮杂邻醌甲基化物和吡啶鎓 1,4-两性离子硫醇盐之间的 [4+3] 环化合成功能化 4,1-苯并硫氮杂
氮杂-邻醌甲基化物和吡啶鎓 1,4-两性离子硫醇盐的 [4+3] 环化已被开发用于在温和和无金属条件下一步合成官能化 2,3-不饱和 4,1-苯并硫氮杂卓. 产生的 4,1-苯并硫氮杂通过m -CPBA 的选择性氧化可以很容易地转化为具有生物学意义的亚砜和砜。
更新日期:2021-10-28
中文翻译:

通过氮杂邻醌甲基化物和吡啶鎓 1,4-两性离子硫醇盐之间的 [4+3] 环化合成功能化 4,1-苯并硫氮杂
氮杂-邻醌甲基化物和吡啶鎓 1,4-两性离子硫醇盐的 [4+3] 环化已被开发用于在温和和无金属条件下一步合成官能化 2,3-不饱和 4,1-苯并硫氮杂卓. 产生的 4,1-苯并硫氮杂通过m -CPBA 的选择性氧化可以很容易地转化为具有生物学意义的亚砜和砜。











































 京公网安备 11010802027423号
京公网安备 11010802027423号