当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(I)-Catalyzed Decarboxylative Arylation of Alkynyl Cyclic Carbonates: Divergent Access to Substituted α-Allenols and 1,3-Butadienes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/adsc.202101064 Chandra Mouleeswara Rao Volla 1 , Geetanjali Sontakke 1 , Rahul Shukla 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/adsc.202101064 Chandra Mouleeswara Rao Volla 1 , Geetanjali Sontakke 1 , Rahul Shukla 1
Affiliation
|
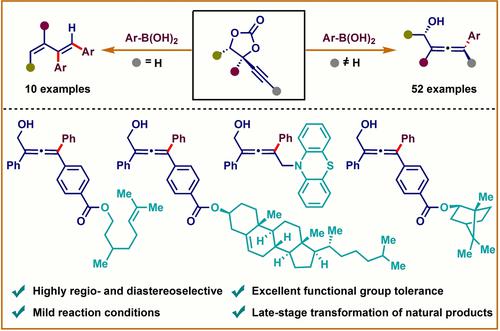
Rh(I)-catalyzed decarboxylative arylation of alkynyl cyclic carbonates using commercially available and low-toxic aryl boronic acids has been disclosed. Depending on the nature of the cyclic carbonates, the methodology provides a straightforward platform to access either substituted 2,3-allenols or 1,3-butadiene derivatives. Internal alkynyl cyclic carbonates undergo monoarylation to conveniently afford 2,3-allenols with high syn-selectivity for the aryl and hydroxy groups. Whereas, terminal alkynyl carbonates led to the formation of diarylated 1,3-butadiene derivatives having cis-configuration for the two aryl groups via allenyl rhodium(I)alkoxide intermediate. The compatibility of various functional groups allowed to develop a library of diversely functionalized scaffolds with excellent regioselectivity in good yields. Late-stage transformation of a series of natural products highlights the wide applicability of the arylation process. Additionally, scale-up experiments and downstream transformations of α-allenol derivatives into other valuable heterocycles illustrate the efficacy of the protocol.
中文翻译:

Rh(I)-催化的炔基环状碳酸酯的脱羧芳基化:取代 α-丙二烯醇和 1,3-丁二烯的不同途径
已经公开了使用可商购的和低毒的芳基硼酸对炔基环状碳酸酯进行Rh(I)催化的脱羧芳基化。根据环状碳酸酯的性质,该方法提供了一个直接的平台来获取取代的 2,3-丙二烯醇或 1,3-丁二烯衍生物。内部炔基环状碳酸酯进行单芳基化以方便地提供对芳基和羟基具有高顺式选择性的2,3-丙二烯醇。然而,末端碳酸炔基酯导致形成对两个芳基具有顺式构型的二芳基化1,3-丁二烯衍生物烯基铑 (I) 醇盐中间体。各种官能团的相容性允许开发具有优异区域选择性和良好产率的多种官能化支架库。一系列天然产物的后期转化凸显了芳基化过程的广泛适用性。此外, α-丙二烯醇衍生物向其他有价值的杂环的放大实验和下游转化说明了该协议的功效。
更新日期:2021-10-28
中文翻译:

Rh(I)-催化的炔基环状碳酸酯的脱羧芳基化:取代 α-丙二烯醇和 1,3-丁二烯的不同途径
已经公开了使用可商购的和低毒的芳基硼酸对炔基环状碳酸酯进行Rh(I)催化的脱羧芳基化。根据环状碳酸酯的性质,该方法提供了一个直接的平台来获取取代的 2,3-丙二烯醇或 1,3-丁二烯衍生物。内部炔基环状碳酸酯进行单芳基化以方便地提供对芳基和羟基具有高顺式选择性的2,3-丙二烯醇。然而,末端碳酸炔基酯导致形成对两个芳基具有顺式构型的二芳基化1,3-丁二烯衍生物烯基铑 (I) 醇盐中间体。各种官能团的相容性允许开发具有优异区域选择性和良好产率的多种官能化支架库。一系列天然产物的后期转化凸显了芳基化过程的广泛适用性。此外, α-丙二烯醇衍生物向其他有价值的杂环的放大实验和下游转化说明了该协议的功效。









































 京公网安备 11010802027423号
京公网安备 11010802027423号