当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring-closing metathesis of N-alkenyl-cyanamides
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-19 , DOI: 10.1039/d1qo01416a Damien F. Dewez 1 , Christina Diacofotaki 1 , Gwilherm Evano 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-19 , DOI: 10.1039/d1qo01416a Damien F. Dewez 1 , Christina Diacofotaki 1 , Gwilherm Evano 1
Affiliation
|
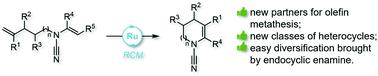
An entry to new classes of nitrogen-containing heterocycles featuring both a cyanamide and an unsaturated 5-, 6- or 7-membered ring is reported. They can be readily obtained by ring-closing metathesis of the corresponding N-alkenyl-cyanamides – new partners for olefin metathesis that can be easily obtained by copper- or palladium-catalyzed alkenylation of cyanamides – using Hoveyda–Grubbs or Grubbs second generation catalysts. In addition to the potential of these novel heterocycles in drug discovery or as building blocks, the presence of the endocyclic double bond was shown to provide a useful access to novel polycyclic heterocycles.
中文翻译:

N-烯基-氰胺的闭环复分解
报道了一种新类别的含氮杂环,其特征是氰胺和不饱和的 5、6 或 7 元环。它们可以通过相应的N-烯基-氰胺的闭环复分解轻松获得 - 烯烃复分解的新伙伴,可以通过铜或钯催化的氰胺的烯基化轻松获得 - 使用 Hoveyda-Grubbs 或 Grubbs 第二代催化剂。除了这些新型杂环在药物发现中或作为构建块的潜力外,内环双键的存在被证明为获得新型多环杂环提供了有用的途径。
更新日期:2021-10-28
中文翻译:

N-烯基-氰胺的闭环复分解
报道了一种新类别的含氮杂环,其特征是氰胺和不饱和的 5、6 或 7 元环。它们可以通过相应的N-烯基-氰胺的闭环复分解轻松获得 - 烯烃复分解的新伙伴,可以通过铜或钯催化的氰胺的烯基化轻松获得 - 使用 Hoveyda-Grubbs 或 Grubbs 第二代催化剂。除了这些新型杂环在药物发现中或作为构建块的潜力外,内环双键的存在被证明为获得新型多环杂环提供了有用的途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号