Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-10-27 , DOI: 10.1016/j.molliq.2021.117928 Yanjun Shen 1 , Xin Wei 1 , Yongzhi Wang 2 , Yutian Shen 3 , Lei Li 4 , Yongli Huang 5 , Kostya Ken Ostrikov 6 , Chang Q Sun 4, 7
|
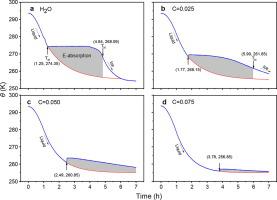
Freezing-thawing cycling of salt solutions is ubiquitously important to fields varying from biochemistry to agriculture, climate, and geological engineering. However, understanding the dynamics of energy exchange and freezing-temperature TN shift during phase transition of the concentrated solutions remains elusive despite intensive investigations since the discovery of Homeister series in 1888. Here we address this issue by focusing on the performance of the hydrogen bond (O:H–O) in the fraction fS molecules of the hydrating supersolid phase and in the remaining fraction fO molecules of the pristine phase during NaCl solution ice formation. The supersolid formation is related to the effect of ionic polarization that shortens and stiffens the H–O bond, while lengthening and weakening the O:H non-bonding interactions in the hydration cells. The supersolid phase is gel-like, less dense, viscoelastic, and mechanically and thermally stable. We demonstrate that the polarization-weakening of the O:H non-bonding interactions of the supersolid phase reduces the TN of the solution and that the H–O cooling contraction in the quasisolid (QS) phase of the pristine water absorbs energy during the Liquid-QS-Ice transition of solution. At fS = 1, neither TN nor energy absorption is resolved within the range of 273 ± 20 K. The least saturation number of molecules is 10 per pair of Na+ and Cl- ions. These findings shall help to develop solutions with tunable TN for practical applications and to understand the mechanism of energy exchange during phase transition in the temperature and time domains.
中文翻译:

冰形成过程中NaCl溶液的能量吸收和冷冻温度可调性
盐溶液的冻融循环对于从生物化学到农业、气候和地质工程的各个领域都非常重要。然而,尽管自 1888 年发现 Homeister 系列以来进行了深入研究,但对浓溶液相变过程中能量交换和冷冻温度 T N位移的动力学仍然难以理解。 在这里,我们通过关注氢键的性能来解决这个问题(O:H-O) 在水合超固相的f S分子部分和剩余部分 f ONaCl 溶液冰形成过程中原始相的分子。超固体的形成与离子极化的影响有关,它缩短和加强了 H-O 键,同时延长和削弱了水合细胞中的 O:H 非键相互作用。超固相是凝胶状的,密度较小,具有粘弹性,并且具有机械和热稳定性。我们证明了超固相的 O:H 非键合相互作用的极化减弱降低了溶液的 T N并且原始水的准固态 (QS) 相中的 H-O 冷却收缩吸收了能量。溶液的液体-QS-冰过渡。在 f S = 1 时,T N在 273 ± 20 K 的范围内也没有能量吸收。最少饱和分子数是每对 Na +和 Cl -离子 10 个。这些发现将有助于为实际应用开发具有可调 T N 的解决方案,并有助于了解温度和时间域中相变过程中的能量交换机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号