Water Research ( IF 11.4 ) Pub Date : 2021-10-27 , DOI: 10.1016/j.watres.2021.117801 Matthew Ross Jones 1 , Bradley M Tebo 1
|
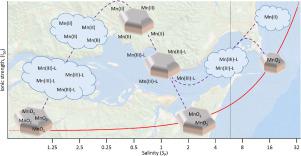
Mixing of waters of different ionic strengths induces the geochemical cycling of reactive elements. The most reactive zone is where the gradient in ionic strength is steepest. In oxygenated systems, the redox-active metal manganese cycles between soluble and particulate fractions through three oxidation states, manganese(II), manganese(III) and manganese(IV). This cycling strongly affects the mobility of inorganic and organic chemicals. The most accessible environmental system where waters with different ionic strengths mix are estuaries. During six Eulerian studies in the Columbia River Estuary, each up to 26 h, we measured manganese speciation and concentration across a salinity (SP) gradient centred around SP = 0.06–6, equivalent to a seawater ionic strength (ISp) of 1.2–120 mM. This zone, representing the region between freshwater and the more intensively studied estuarine turbidity maximum, presents a highly dynamic geochemical environment in which the manganese cycle propagates through four steps as ISp increases due to mixing: 1. Before a measurable change in ISp, manganese, as particulate manganese(III/IV) oxides (MnOx), undergoes reduction, independent of photochemical processes, to soluble manganese(III) stabilized in organic complexes (Mn(III)-L) and manganese(II); 2. As ISp increases between 5 and 80 mM, Mn(III)-L reduction continues and manganese(II) adsorbs onto particle surfaces; 3. As ISp increases further, though remaining below 80 mM (SP ≈ 4), adsorbed manganese(II) desorbs and/or is oxidized and is released as Mn(III)-L or oxidises further to MnOx; 4. The breakdown of Mn(III)-L complexes leads to higher manganese(II) and MnOx, which at Mid-Estuary-Salinities (ISp = 320–480 mM) precipitates. This manganese cycling in low ISp waters directly affects a system's redox chemistry and provides a window into understanding the extensive, yet hidden, freshwater/saline water interface in aquifers, soils, sediments and estuaries.
中文翻译:

哥伦比亚河口中离子强度非常低的新型锰循环
不同离子强度的水的混合会引起活性元素的地球化学循环。最具反应性的区域是离子强度梯度最陡峭的区域。在含氧系统中,氧化还原活性金属锰通过三种氧化态锰 (II)、锰 (III) 和锰 (IV) 在可溶部分和颗粒部分之间循环。这种循环强烈影响无机和有机化学品的流动性。具有不同离子强度的水混合的最容易接近的环境系统是河口。期间,在哥伦比亚河口6个欧拉研究,每个多达26小时,我们测量锰形态和浓度跨越盐度(小号P)为中心的梯度围绕小号P = 0.06-6,相当于海水的离子强度(I小号p 1.2-120毫)。该区域中,表示淡水和更深入研究河口最大浑浊之间的区域中,呈现出高度动态的地球化学环境,其中通过四个步骤的锰周期传播作为我小号p增加由于混合:1.在我的可测量的变化之前小号p,锰,作为颗粒状锰 (III/IV) 氧化物 (MnO x ),在独立于光化学过程的情况下被还原为稳定在有机复合物 (Mn(III)-L) 和锰 (II) 中的可溶性锰 (III);2.如我小号p在 5 到 80 mM 之间增加,Mn(III)-L 还原继续并且锰 (II) 吸附到颗粒表面;3.如我小号p进一步增加,尽管剩余的低于80毫米(小号P ≈4),吸附锰(II)解吸和/或氧化和被释放的Mn(III)-L或中氧化进一步的MnO X ; 4.锰(III)的击穿-L络合物导致更高的锰(II)和MnO X,其在中部河口-盐度(I小号p = 320-480毫米)沉淀物。这种锰循环在低 I S p 水直接影响系统的氧化还原化学,并为了解含水层、土壤、沉积物和河口中广泛但隐藏的淡水/盐水界面提供了一个窗口。











































 京公网安备 11010802027423号
京公网安备 11010802027423号