当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Density and Viscosity of Aniline and 1-Alkanol Binary Mixtures
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.jced.1c00491 Mohammad Almasi 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.jced.1c00491 Mohammad Almasi 1
Affiliation
|
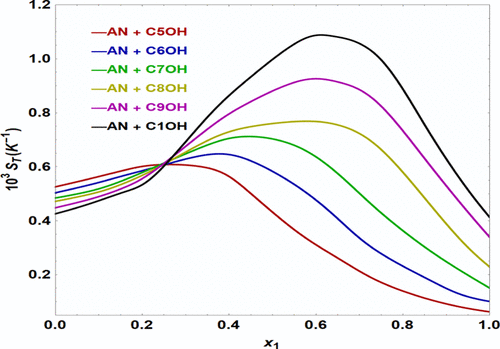
In this study, values of the density and viscosity of aniline and normal alkanol (1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol) were measured at 293.15–323.15 K. The governing molecular forces in the mixtures were discussed by two important parameters, namely, the mutual diffusion coefficient D12 and separation ratio ψ. Values of the separation ratio are negative for all binary fluids and increase with the CH2 groups of alcohols. Findings indicate that increasing the alcohol chain length increases its movement and reduces its interaction with aniline molecules. The perturbed chain statistical associating fluid model was utilized to correlate the mixture densities at different temperatures. The consistency among the experimental and predicted values is reasonable for all binary mixtures.
中文翻译:

苯胺和 1-烷醇二元混合物的实验密度和粘度
在本研究中,苯胺和正链烷醇(1-戊醇、1-己醇、1-庚醇、1-辛醇、1-壬醇和 1-癸醇)的密度和粘度值在 293.15-323.15 K 下测量。控制混合物中的分子力由两个重要参数讨论,即相互扩散系数D 12和分离比 ψ。所有二元流体的分离比值为负,并随着 CH 2 的增加而增加醇类。研究结果表明,增加醇链长度会增加其运动并减少其与苯胺分子的相互作用。扰动链统计关联流体模型用于关联不同温度下的混合物密度。对于所有二元混合物,实验值和预测值之间的一致性是合理的。
更新日期:2021-12-09
中文翻译:

苯胺和 1-烷醇二元混合物的实验密度和粘度
在本研究中,苯胺和正链烷醇(1-戊醇、1-己醇、1-庚醇、1-辛醇、1-壬醇和 1-癸醇)的密度和粘度值在 293.15-323.15 K 下测量。控制混合物中的分子力由两个重要参数讨论,即相互扩散系数D 12和分离比 ψ。所有二元流体的分离比值为负,并随着 CH 2 的增加而增加醇类。研究结果表明,增加醇链长度会增加其运动并减少其与苯胺分子的相互作用。扰动链统计关联流体模型用于关联不同温度下的混合物密度。对于所有二元混合物,实验值和预测值之间的一致性是合理的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号