当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ-Formed Phenoxyl Radical on the CuO Surface Triggers Efficient Persulfate Activation for Phenol Degradation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.est.1c03758 Mingjie Huang 1, 2 , Yi Han 1 , Wei Xiang 1, 3 , Delai Zhong 4 , Chen Wang 1, 3 , Tao Zhou 1 , Xiaohui Wu 1, 3 , Juan Mao 1, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.est.1c03758 Mingjie Huang 1, 2 , Yi Han 1 , Wei Xiang 1, 3 , Delai Zhong 4 , Chen Wang 1, 3 , Tao Zhou 1 , Xiaohui Wu 1, 3 , Juan Mao 1, 3
Affiliation
|
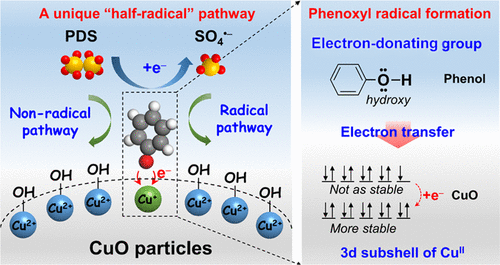
Transition-metal oxide (MxOy)-based persulfate (PDS) activation processes have demonstrated enormous potential for pollutant degradation in water purification. However, the mechanistic insight of PDS activation by a MxOy catalyst concerning the mediate role of the organic substrate remains obscure. Here, we demonstrated that the in situ-formed phenoxyl radical on the CuO surface can trigger efficient persulfate activation for phenol degradation. The formation of the phenoxyl radical was an inner-sphere process, which involved the successive steps of chemisorption through surface hydroxyl group substitution and the subsequent spontaneous electron transfer reaction from adsorbed phenol to CuO. The organic substrate phenol can be oxidized by the PDS molecule and surface-bound SO4•– through the nonradical and free-radical pathways, respectively. Such a unique “half-radical” mechanism resulted in an extraordinarily high PDS utilization efficiency of 188.9%. More importantly, a general rule for phenoxyl radical formation was concluded; it can be formed in the cases of organic substrates with a Hammett constant σ+ lower than −0.02 and metal ion of a 3d subshell between half-filled and fully filled. This study clarifies the mediate role of the organic substrate for interfacial PDS activation on MxOy and also gives new insights into the rational design of a highly efficient MxOy catalyst for selective phenolic/aniline pollutant degradation in wastewater.
中文翻译:

CuO 表面原位形成的苯氧基自由基触发有效的过硫酸盐活化以降解苯酚
基于过渡金属氧化物 (M x O y ) 的过硫酸盐 (PDS) 活化过程已显示出在水净化中降解污染物的巨大潜力。然而,M x O y对 PDS 激活的机制洞察关于有机底物的中介作用的催化剂仍然不清楚。在这里,我们证明了在 CuO 表面原位形成的苯氧基自由基可以触发有效的过硫酸盐活化以降解苯酚。苯氧基自由基的形成是一个内球过程,包括通过表面羟基取代的化学吸附和随后从吸附的苯酚到 CuO 的自发电子转移反应的连续步骤。有机底物苯酚可以被 PDS 分子和表面结合的 SO 4氧化•–分别通过非自由基和自由基途径。这种独特的“半自由基”机制导致了 188.9% 的异常高的 PDS 利用效率。更重要的是,得出了苯氧基自由基形成的一般规律;它可以在哈米特常数 σ +低于 -0.02的有机基板和半填充和完全填充之间的 3d 亚壳的金属离子的情况下形成。该研究阐明了有机底物对 M x O y界面 PDS 活化的中介作用,并为高效 M x O y催化剂的合理设计提供了新的见解,用于选择性降解废水中的酚类/苯胺污染物。
更新日期:2021-11-16
中文翻译:

CuO 表面原位形成的苯氧基自由基触发有效的过硫酸盐活化以降解苯酚
基于过渡金属氧化物 (M x O y ) 的过硫酸盐 (PDS) 活化过程已显示出在水净化中降解污染物的巨大潜力。然而,M x O y对 PDS 激活的机制洞察关于有机底物的中介作用的催化剂仍然不清楚。在这里,我们证明了在 CuO 表面原位形成的苯氧基自由基可以触发有效的过硫酸盐活化以降解苯酚。苯氧基自由基的形成是一个内球过程,包括通过表面羟基取代的化学吸附和随后从吸附的苯酚到 CuO 的自发电子转移反应的连续步骤。有机底物苯酚可以被 PDS 分子和表面结合的 SO 4氧化•–分别通过非自由基和自由基途径。这种独特的“半自由基”机制导致了 188.9% 的异常高的 PDS 利用效率。更重要的是,得出了苯氧基自由基形成的一般规律;它可以在哈米特常数 σ +低于 -0.02的有机基板和半填充和完全填充之间的 3d 亚壳的金属离子的情况下形成。该研究阐明了有机底物对 M x O y界面 PDS 活化的中介作用,并为高效 M x O y催化剂的合理设计提供了新的见解,用于选择性降解废水中的酚类/苯胺污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号