当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Based Dual Active Site-Mediated Peroxymonosulfate Activation for the Degradation of Emerging Organic Pollutants
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.est.1c06205 Shizong Wang 1, 2 , Lejin Xu 3 , Jianlong Wang 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.est.1c06205 Shizong Wang 1, 2 , Lejin Xu 3 , Jianlong Wang 1, 2
Affiliation
|
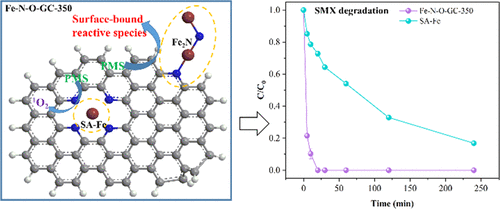
It is still a challenge to synthesize highly efficient and stable catalysts for the Fenton-like reaction. In this study, we constructed an integrated catalyst with highly dispersed iron-based dual active sites, in which Fe2N and single-atom Fe (SA-Fe) were embedded into nitrogen- and oxygen-co-doped graphitic carbon (Fe-N-O-GC-350). Extended X-ray absorption fine structure (EXAFS) confirmed the coordination structure of iron, and line combination fitting (LCF) demonstrated the coexistence of Fe2N and SA-Fe with percentages of 75 and 25%, respectively. Iron-based dual active sites endowed Fe-N-O-GC-350 with superior catalytic activity to activate peroxymonosulfate (PMS) as evidenced by the fast degradation rate of sulfamethoxazole (SMX) (0.24 min–1) in the presence of 0.4 mM PMS and 0.1 g/L Fe-N-O-GC-350. Unlike the reported singlet oxygen and high-valent iron oxo-mediated degradation induced by the SA-Fe catalyst, both surface-bound reactive species and singlet oxygen contributed to SMX degradation, while surface-bound reactive species dominated. Density functional theory (DFT) simulation indicated that Fe2N and SA-Fe enhanced the adsorption of PMS, which played a key role in PMS activation. The Fe-N-O-GC-350/PMS system had resistance to the interference of common inorganic anions and high oxidation capacity to recalcitrant organic contaminants. This study elucidated the important role of Fe2N in PMS activation and provide a clue to design rationally catalysts with iron-based dual active sites to activate PMS for the degradation of emerging organic pollutants.
中文翻译:

用于降解新兴有机污染物的铁基双活性位点介导过硫酸盐活化
合成高效稳定的类芬顿反应催化剂仍然是一个挑战。在这项研究中,我们构建了一种具有高度分散的铁基双活性位点的集成催化剂,其中 Fe 2 N 和单原子 Fe (SA-Fe) 嵌入到氮和氧共掺杂的石墨碳 (Fe- NO-GC-350)。扩展 X 射线吸收精细结构 (EXAFS) 证实了铁的配位结构,线组合拟合 (LCF) 证明了 Fe 2 N 和 SA-Fe的共存比例分别为 75% 和 25%。铁基双活性位点赋予 Fe-NO-GC-350 优异的催化活性来活化过硫酸盐 (PMS),磺胺甲恶唑 (SMX) 的快速降解速率 (0.24 min –1 ) 证明了这一点) 在 0.4 mM PMS 和 0.1 g/L Fe-NO-GC-350 存在下。与报道的由 SA-Fe 催化剂诱导的单线态氧和高价铁氧介导的降解不同,表面结合的活性物质和单线态氧都有助于 SMX 降解,而表面结合的活性物质占主导地位。密度泛函理论 (DFT) 模拟表明 Fe 2 N 和 SA-Fe 增强了 PMS 的吸附,这在 PMS 活化中起关键作用。Fe-NO-GC-350/PMS 系统具有抗常见无机阴离子的干扰和对顽固有机污染物的高氧化能力。本研究阐明了 Fe 2PMS 活化中的 N 并为合理设计具有铁基双活性位点的催化剂以激活 PMS 降解新兴有机污染物提供线索。
更新日期:2021-11-16
中文翻译:

用于降解新兴有机污染物的铁基双活性位点介导过硫酸盐活化
合成高效稳定的类芬顿反应催化剂仍然是一个挑战。在这项研究中,我们构建了一种具有高度分散的铁基双活性位点的集成催化剂,其中 Fe 2 N 和单原子 Fe (SA-Fe) 嵌入到氮和氧共掺杂的石墨碳 (Fe- NO-GC-350)。扩展 X 射线吸收精细结构 (EXAFS) 证实了铁的配位结构,线组合拟合 (LCF) 证明了 Fe 2 N 和 SA-Fe的共存比例分别为 75% 和 25%。铁基双活性位点赋予 Fe-NO-GC-350 优异的催化活性来活化过硫酸盐 (PMS),磺胺甲恶唑 (SMX) 的快速降解速率 (0.24 min –1 ) 证明了这一点) 在 0.4 mM PMS 和 0.1 g/L Fe-NO-GC-350 存在下。与报道的由 SA-Fe 催化剂诱导的单线态氧和高价铁氧介导的降解不同,表面结合的活性物质和单线态氧都有助于 SMX 降解,而表面结合的活性物质占主导地位。密度泛函理论 (DFT) 模拟表明 Fe 2 N 和 SA-Fe 增强了 PMS 的吸附,这在 PMS 活化中起关键作用。Fe-NO-GC-350/PMS 系统具有抗常见无机阴离子的干扰和对顽固有机污染物的高氧化能力。本研究阐明了 Fe 2PMS 活化中的 N 并为合理设计具有铁基双活性位点的催化剂以激活 PMS 降解新兴有机污染物提供线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号