当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Wanzlick's equilibrium in tri- and tetraaminoolefins
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-14 , DOI: 10.1039/d1qo01320c Julian Messelberger 1 , Manoj Kumar 1 , Stephen J. Goodner 1, 2 , Dominik Munz 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-14 , DOI: 10.1039/d1qo01320c Julian Messelberger 1 , Manoj Kumar 1 , Stephen J. Goodner 1, 2 , Dominik Munz 1, 2
Affiliation
|
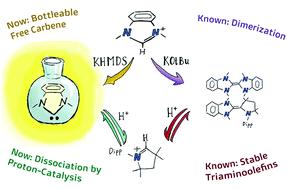
The dissociation mechanism of electron-rich olefins into their parent carbenes has been a controversial topic since Wanzlick's pioneering work. Herein, we present a combined synthetic and computational study on the formation (dissociation, respectively) of hetero- and homo-carbene dimers derived from benzimidazolin-2-ylidenes (benzNHCs), imidazolidin-2-ylidenes (saNHC), and cyclic (alkyl) (amino) carbenes (CAACs) through sublimation (in vacuo) as well as in condensed phase. We quantify the effect of proton catalysis and report that even triaminoolefins dissociate to their free carbenes, yet only under proton catalysis. Accordingly, we report how the judicious choice of the base (KOtBu vs. KHMDS) and solvent (hexane/benzene vs. THF) allows N,N′-dimethylbenzimidazolin-2-ylidene to be obtained quantitatively as a metastable, kinetic product. This free carbene had been previously reported to dimerize directly to the olefin-dimer, which is the thermodynamic product.
中文翻译:

Wanzlick 在三和四氨基烯烃中的平衡
自 Wanzlick 的开创性工作以来,富电子烯烃的解离机制成为其母体卡宾一直是一个有争议的话题。在此,我们对衍生自苯并咪唑啉-2-亚基(benzNHCs)、咪唑啉-2-亚基(saNHC)和环状(烷基)(氨基)卡宾(CAAC)通过升华(真空)以及凝聚相。我们量化了质子催化的影响,并报告说即使是三氨基烯烃也能解离为其游离卡宾,但仅在质子催化下。因此,我们报告了碱(KO t Bu vs. KHMDS)和溶剂(己烷/苯vs. KHMDS)的明智选择。THF) 允许定量获得N , N '-二甲基苯并咪唑啉-2-亚基,作为亚稳态动力学产物。先前已报道这种游离卡宾直接二聚成烯烃二聚体,即热力学产物。
更新日期:2021-10-26
中文翻译:

Wanzlick 在三和四氨基烯烃中的平衡
自 Wanzlick 的开创性工作以来,富电子烯烃的解离机制成为其母体卡宾一直是一个有争议的话题。在此,我们对衍生自苯并咪唑啉-2-亚基(benzNHCs)、咪唑啉-2-亚基(saNHC)和环状(烷基)(氨基)卡宾(CAAC)通过升华(真空)以及凝聚相。我们量化了质子催化的影响,并报告说即使是三氨基烯烃也能解离为其游离卡宾,但仅在质子催化下。因此,我们报告了碱(KO t Bu vs. KHMDS)和溶剂(己烷/苯vs. KHMDS)的明智选择。THF) 允许定量获得N , N '-二甲基苯并咪唑啉-2-亚基,作为亚稳态动力学产物。先前已报道这种游离卡宾直接二聚成烯烃二聚体,即热力学产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号