Water Research ( IF 11.4 ) Pub Date : 2021-10-26 , DOI: 10.1016/j.watres.2021.117802 Jia Deng 1 , Enlai Gao 2 , Feng Wu 3 , Zhixiong You 3 , Xiaozhong Li 3 , Shuxian Gao 4 , Li-Zhi Huang 1
|
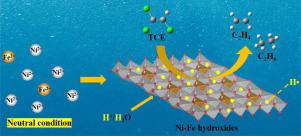
Atomic hydrogen (H•) is highly reactive for the hydrodechlorination of trichloroethylene (TCE). In this work, we found that the coprecipitation of Ni2+ and Fe2+ at neutral pH led to an unprecedented catalytic generation of H•. The generated H• effectively dechlorinate TCE to nontoxic ethylene and ethane, and Fe2+ is the only electron donor. The abundant adsorbed H• produced with a Ni/Fe ratio of 0.4 enhances hydrogen evolution reaction causing a low efficiency for hydrodechlorination. In contrast, the active absorbed H• is generated in the crystal lattice of Ni-Fe hydroxides with a Ni/Fe ratio of 3.0 causing highly efficient hydrodechlorination of TCE. This work not only reveals the mechanism of catalytic hydrodechlorination by Ni-Fe hydroxides at neutral pH, but also provides a novel approach to detoxify TCE in contaminated water using facile precipitated Ni-Fe hydroxides.
中文翻译:

Ni-Fe氢氧化物产生原子氢:三氯乙烯加氢脱氯的机理和活性
原子氢 (H•) 对三氯乙烯 (TCE) 的加氢脱氯具有高度反应性。在这项工作中,我们发现 Ni 2+和 Fe 2+在中性 pH 值下的共沉淀导致了前所未有的 H• 催化生成。生成的 H• 有效地将 TCE 脱氯为无毒的乙烯和乙烷,以及 Fe 2+是唯一的电子供体。Ni/Fe 比为 0.4 时产生的大量吸附 H• 会增强析氢反应,导致加氢脱氯效率低下。相比之下,活性吸收的 H• 在 Ni-Fe 氢氧化物的晶格中产生,Ni/Fe 比为 3.0,从而导致 TCE 的高效加氢脱氯。这项工作不仅揭示了 Ni-Fe 氢氧化物在中性 pH 值下催化加氢脱氯的机理,而且还提供了一种使用易沉淀的 Ni-Fe 氢氧化物对污染水中的 TCE 进行解毒的新方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号