当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-catalyzed C(sp2)–N-bond coupling of boronic acids and cyclic imides
Chemical Communications ( IF 4.3 ) Pub Date : 2021-10-19 , DOI: 10.1039/d1cc04356k Linn Neerbye Berntsen 1 , Thomas Nordbø Solvi 1 , Kristian Sørnes 1 , David S Wragg 1 , Alexander H Sandtorv 1
Chemical Communications ( IF 4.3 ) Pub Date : 2021-10-19 , DOI: 10.1039/d1cc04356k Linn Neerbye Berntsen 1 , Thomas Nordbø Solvi 1 , Kristian Sørnes 1 , David S Wragg 1 , Alexander H Sandtorv 1
Affiliation
|
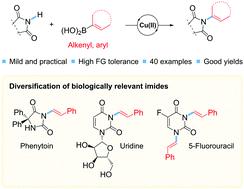
A general Cu-catalyzed strategy for coupling cyclic imides and alkenylboronic acids by forming C(sp2)–N-bonds is reported. The method enables the practical and mild preparation of (E)-enimides. A large range of cyclic imides are allowed, and di- and tri-substituted alkenylboronic acids can be used. Full retention was observed in the configuration of the alkene double bond in the coupled products. The method is also applicable for preparing N-arylimides, using arylboronic acids as coupling partners. The usefulness of this strategy is exemplified by the convenient derivatization of the chemotherapy medication 5-flurouracil, the nucleoside uridine and the anti-epileptic drug phenytoin.
中文翻译:

Cu催化的硼酸和环状酰亚胺的C(sp2)-N键偶联
报道了通过形成 C(sp 2 ) - N-键来偶联环状酰亚胺和烯基硼酸的一般铜催化策略。该方法能够实现(E)-烯酰亚胺的实用且温和的制备。允许使用大范围的环状酰亚胺,并且可以使用二取代和三取代的烯基硼酸。在偶联产物中烯烃双键的构型中观察到完全保留。该方法也适用于以芳基硼酸为偶联剂制备N-芳基酰亚胺。化疗药物 5-氟尿嘧啶、核苷尿苷和抗癫痫药物苯妥英的方便衍生化证明了该策略的有效性。
更新日期:2021-10-26
中文翻译:

Cu催化的硼酸和环状酰亚胺的C(sp2)-N键偶联
报道了通过形成 C(sp 2 ) - N-键来偶联环状酰亚胺和烯基硼酸的一般铜催化策略。该方法能够实现(E)-烯酰亚胺的实用且温和的制备。允许使用大范围的环状酰亚胺,并且可以使用二取代和三取代的烯基硼酸。在偶联产物中烯烃双键的构型中观察到完全保留。该方法也适用于以芳基硼酸为偶联剂制备N-芳基酰亚胺。化疗药物 5-氟尿嘧啶、核苷尿苷和抗癫痫药物苯妥英的方便衍生化证明了该策略的有效性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号