当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric cross-aldol reactions of α-keto hydrazones and α,β-unsaturated γ-keto hydrazones with trifluoromethyl ketones
Chemical Communications ( IF 4.3 ) Pub Date : 2021-10-15 , DOI: 10.1039/d1cc05014a Saúl Alberca 1 , Esteban Matador 1 , Javier Iglesias-Sigüenza 1 , Ma de Gracia Retamosa 1, 2 , Rosario Fernández 1 , José M Lassaletta 3 , David Monge 1
Chemical Communications ( IF 4.3 ) Pub Date : 2021-10-15 , DOI: 10.1039/d1cc05014a Saúl Alberca 1 , Esteban Matador 1 , Javier Iglesias-Sigüenza 1 , Ma de Gracia Retamosa 1, 2 , Rosario Fernández 1 , José M Lassaletta 3 , David Monge 1
Affiliation
|
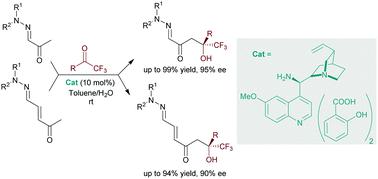
α-Keto hydrazones and α,β-unsaturated γ-keto hydrazones are suitable pro-nucleophiles for asymmetric cross-aldol reactions with trifluoromethyl ketones via aza-di(tri)enamine-type intermediates. A quinidine-derived primary amine catalyst affords tertiary trifluoromethylated alcohols in good-to-excellent yields and high enantioselectivities. Subsequent transformations of hydrazono moieties yield appealing fluorinated carboxylic acids, 1,4-dicarbonyls and γ-keto acids.
中文翻译:

α-酮腙和α,β-不饱和γ-酮腙与三氟甲基酮的不对称交叉羟醛反应
α-酮腙和α,β-不饱和γ-酮腙是合适的亲核试剂,用于通过氮杂二(三)烯胺型中间体与三氟甲基酮进行不对称交叉羟醛反应。奎尼丁衍生的伯胺催化剂以良好的收率和高对映选择性提供三氟甲基化叔醇。腙部分的后续转化产生了吸引人的氟化羧酸、1,4-二羰基和γ-酮酸。
更新日期:2021-10-26
中文翻译:

α-酮腙和α,β-不饱和γ-酮腙与三氟甲基酮的不对称交叉羟醛反应
α-酮腙和α,β-不饱和γ-酮腙是合适的亲核试剂,用于通过氮杂二(三)烯胺型中间体与三氟甲基酮进行不对称交叉羟醛反应。奎尼丁衍生的伯胺催化剂以良好的收率和高对映选择性提供三氟甲基化叔醇。腙部分的后续转化产生了吸引人的氟化羧酸、1,4-二羰基和γ-酮酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号