当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroquinone-Based Synthesis of Pd Nanostructures and the Interplay of Surface Capping, Reduction Kinetics, Attachment, Diffusion, and Fusion
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-10-22 , DOI: 10.1021/acs.chemmater.1c02791 Anderson G. M. da Silva 1, 2 , Ruhui Chen 3 , Quynh N. Nguyen 3 , Madeline Vara 3 , Pedro H. C. Camargo 4 , Younan Xia 1, 3
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-10-22 , DOI: 10.1021/acs.chemmater.1c02791 Anderson G. M. da Silva 1, 2 , Ruhui Chen 3 , Quynh N. Nguyen 3 , Madeline Vara 3 , Pedro H. C. Camargo 4 , Younan Xia 1, 3
Affiliation
|
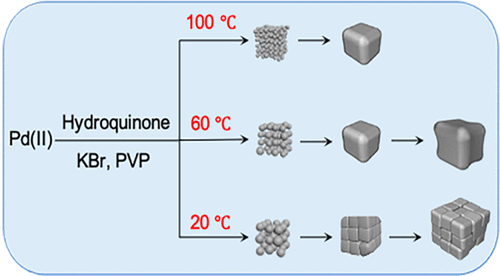
The rational synthesis of nanostructured materials with desired properties calls for a thorough understanding of the growth mechanism. Here we report a mechanistic case study of Pd nanostructures synthesized by reducing a Pd(II) precursor with hydroquinone in the presence of KBr. As the reaction temperature was decreased from 100 to 20 °C, sub-10 nm cubes, concave nanocubes, and cube-like aggregates of much smaller particles were sequentially obtained. Our time-lapse experiments and a set of controls indicated that primary particles of 1–4 nm in size were formed during the initial stage of the synthesis, followed by their aggregation into cube-like structures through an attachment process. In addition to the influence of surface capping, the reaction temperature played a vital role in determining the exact shape or morphology of the final products by affecting the reduction kinetics, fusion of the attached particles, and surface diffusion of atoms. At 100 °C, corresponding to a quick depletion of the Pd(II) precursor, the primary particles in each aggregate could easily fuse together to form nanocubes with flat faces owing to adequate surface diffusion. At 60 °C, the constituent particles also fused into cubes and then evolved into concave cubes through atomic deposition at the corners. At 20 °C, although fusion did not occur due to the substantially decreased diffusion rate, the primary particles in each aggregate still grew through atomic deposition for the formation of larger, cube-like aggregates. Explicating the growth mechanism, this work offers a mechanistic understanding of the nonclassical growth mode involved in the formation of various metal nanostructures.
中文翻译:

基于对苯二酚的 Pd 纳米结构的合成以及表面覆盖、还原动力学、附着、扩散和融合的相互作用
具有所需特性的纳米结构材料的合理合成需要对生长机制有透彻的了解。在这里,我们报告了通过在 KBr 存在下用氢醌还原 Pd(II) 前体合成的 Pd 纳米结构的机械案例研究。随着反应温度从 100°C 降低到 20°C,依次获得亚 10 nm 立方体、凹面纳米立方体和小得多的立方体状聚集体。我们的延时实验和一组对照表明,在合成的初始阶段形成了 1-4 nm 大小的初级粒子,然后通过附着过程将它们聚集成立方体状结构。除了表面封盖的影响,反应温度通过影响还原动力学、附着粒子的融合和原子的表面扩散,在确定最终产品的确切形状或形态方面起着至关重要的作用。在 100 °C 时,对应于 Pd(II) 前驱体的快速消耗,由于足够的表面扩散,每个聚集体中的初级粒子可以很容易地融合在一起形成具有平面的纳米立方体。在 60 °C 时,组成粒子也融合成立方体,然后通过角落处的原子沉积演变成凹立方体。在 20 °C 时,虽然由于扩散速率显着降低而没有发生融合,但每个聚集体中的初级粒子仍然通过原子沉积生长,以形成更大的立方体状聚集体。解释增长机制,
更新日期:2021-11-09
中文翻译:

基于对苯二酚的 Pd 纳米结构的合成以及表面覆盖、还原动力学、附着、扩散和融合的相互作用
具有所需特性的纳米结构材料的合理合成需要对生长机制有透彻的了解。在这里,我们报告了通过在 KBr 存在下用氢醌还原 Pd(II) 前体合成的 Pd 纳米结构的机械案例研究。随着反应温度从 100°C 降低到 20°C,依次获得亚 10 nm 立方体、凹面纳米立方体和小得多的立方体状聚集体。我们的延时实验和一组对照表明,在合成的初始阶段形成了 1-4 nm 大小的初级粒子,然后通过附着过程将它们聚集成立方体状结构。除了表面封盖的影响,反应温度通过影响还原动力学、附着粒子的融合和原子的表面扩散,在确定最终产品的确切形状或形态方面起着至关重要的作用。在 100 °C 时,对应于 Pd(II) 前驱体的快速消耗,由于足够的表面扩散,每个聚集体中的初级粒子可以很容易地融合在一起形成具有平面的纳米立方体。在 60 °C 时,组成粒子也融合成立方体,然后通过角落处的原子沉积演变成凹立方体。在 20 °C 时,虽然由于扩散速率显着降低而没有发生融合,但每个聚集体中的初级粒子仍然通过原子沉积生长,以形成更大的立方体状聚集体。解释增长机制,











































 京公网安备 11010802027423号
京公网安备 11010802027423号