International Journal of Cardiology ( IF 3.2 ) Pub Date : 2021-10-23 , DOI: 10.1016/j.ijcard.2021.10.138 Arturo Cesaro 1 , Felice Gragnano 1 , Paolo Calabrò 1 , Elisabetta Moscarella 1 , Francesco Santelli 2 , Fabio Fimiani 3 , Giuseppe Patti 4 , Ilaria Cavallari 5 , Emilia Antonucci 6 , Plinio Cirillo 7 , Pasquale Pignatelli 8 , Gualtiero Palareti 6 , Francesco Pelliccia 9 , Eduardo Bossone 10 , Vittorio Pengo 11 , Paolo Gresele 12 , Rossella Marcucci 13 ,
|
Aim
To analyze the prevalence and clinical implications of the eligibility criteria for prolonged dual antithrombotic therapy with ticagrelor 60 mg twice daily and/or rivaroxaban 2.5 mg twice daily in a contemporary real-world ACS registry.
Methods
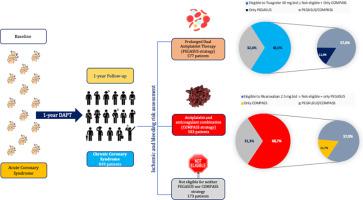
Patients from the START-ANTIPLATELET registry (NCT02219984) were stratified according to the eligibility criteria of the PEGASUS and COMPASS studies to investigate the proportion of patients eligible for prolonged dual antithrombotic therapy at discharge and after 1-year of DAPT. Net adverse clinical events (NACE), defined as all-cause death, myocardial infarction, stroke, and major bleeding, at 1 year were also evaluated and compared among groups.
Results
1844 were considered for the analysis at baseline. Out of 849 event-free patients continually receiving dual antiplatelet therapy for at least 1 year, 577 (68%) and 583 (68.7%) met at least one eligibility criterion for ticagrelor and rivaroxaban, respectively. In the PEGASUS-like patients, age was the most common criterion (71% of cases). The presence ≥2 cardiovascular risk factors was the most common eligibility criterion in the COMPASS-like patients (80.8%). At 1-year follow-up, 211 (11.4%) and 119 (6.5%) patients experienced NACE and MACE, respectively. The incidence of NACEs was higher in the PEGASUS-only group (15.4% vs. 8.4%; p = 0.008) and numerically higher in the COMPASS-only group (10.9% vs. 8.4%; p = 0.299).
Conclusions
In a contemporary real-world ACS cohort, approximately two-thirds of patients that complete 1-year DAPT met the eligibility criteria for ticagrelor 60 mg twice daily or rivaroxaban 2.5 mg twice daily, showing a higher risk of NACEs.
中文翻译:

PEGASUS 和 COMPASS 表型患者延长双重抗血栓治疗资格标准的患病率和临床意义:来自 START-ANTIPLATELET 登记的见解
目标
在当代真实世界的 ACS 登记中,分析替格瑞洛 60 mg 每日两次和/或利伐沙班 2.5 mg 每日两次延长双重抗血栓治疗的资格标准的流行率和临床意义。
方法
来自 START-ANTIPLATELET 注册 (NCT02219984) 的患者根据 PEGASUS 和 COMPASS 研究的资格标准进行分层,以调查出院时和 DAPT 1 年后符合长期双重抗血栓治疗的患者比例。净不良临床事件 (NACE),定义为全因死亡、心肌梗死、卒中和大出血,在 1 年也进行了评估和组间比较。
结果
1844 被考虑用于基线分析。在连续接受双联抗血小板治疗至少 1 年的 849 名无事件患者中,分别有 577 名(68%)和 583 名(68.7%)符合替格瑞洛和利伐沙班的至少一项合格标准。在 PEGASUS 样患者中,年龄是最常见的标准(71% 的病例)。存在≥2个心血管危险因素是COMPASS样患者最常见的合格标准(80.8%)。在 1 年的随访中,分别有 211 名 (11.4%) 和 119 名 (6.5%) 患者经历了 NACE 和 MACE。仅 PEGASUS 组的 NACE 发生率较高(15.4% 对 8.4%;p = 0.008),仅使用 COMPASS 组的 NACE 发生率较高(10.9% 对 8.4%;p = 0.299)。
结论
在当代真实世界的 ACS 队列中,大约三分之二完成 1 年 DAPT 的患者符合替格瑞洛 60 mg 每日两次或利伐沙班 2.5 mg 每日两次的资格标准,显示 NACE 风险较高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号