Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-10-23 , DOI: 10.1016/j.jhazmat.2021.127585 Xin Sun 1 , Minlin Mao 2 , Kaibin Lu 2 , Qimei Hu 2 , Weizhen Liu 1 , Zhang Lin 3
|
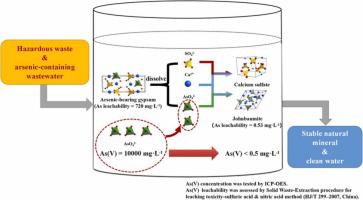
High-level arsenic-containing wastewater (HAW) causes serious environmental pollution. Chemical precipitation is the most widely used technology for treating HAW. However, chemical precipitation generates huge amounts of hazardous solid wastes, which leads to secondary pollution. In this work, an efficient method, producing no secondary pollution was developed for one-step complete removal of As(V) from HAW using a hazardous solid waste namely arsenic-bearing gypsum (ABG). After the treatment, ABG was transformed into highly stable and environment-friendly mineral Johnbaumite. Meanwhile, the arsenic concentration in the wastewater decreased from 10,000 mg·L-1 to 0.22 mg·L-1 under optimized hydrothermal conditions (ABG dosage of 50 g·L-1, solution pH of 13.5, temperature of 150 °C for 12 h). The mechanism mainly included the following processes. (i) The phase transformation of ABG resulted in the release of calcium and hydrogen arsenate ions in ABG. (ii) Hydrogen arsenate ions transformed into arsenate ions in alkaline environment. (iii) Under alkaline conditions, calcium ions combined with arsenate ions to form Johnbaumite, whereas the hydrothermal conditions accelerated the crystal growth of Johnbaumite. This study provides a new idea for the synchronous treatment of toxic heavy metal-containing wastewaters and hazardous solid wastes.
中文翻译:

含砷石膏一步法去除废水中高浓度砷形成约翰鲍姆石
高浓度含砷废水(HAW)造成严重的环境污染。化学沉淀法是处理HAW最广泛使用的技术。然而,化学沉淀会产生大量有害固体废物,造成二次污染。在这项工作中,开发了一种不产生二次污染的有效方法,使用含砷石膏 (ABG) 等有害固体废物一步完全去除 HAW 中的 As(V)。经过处理,ABG 转化为高度稳定的环保矿物约翰鲍姆石。同时,在优化的水热条件(ABG用量为50 g · L -1 , 溶液 pH 值为 13.5, 温度为 150 °C 12 h)。该机制主要包括以下过程。(i) ABG 的相变导致 ABG 中钙离子和砷酸氢离子的释放。(ii) 砷酸根离子在碱性环境中转化为砷酸根离子。(iii) 在碱性条件下,钙离子与砷酸根离子结合形成约翰鲍姆石,而水热条件加速了约翰鲍姆石的晶体生长。该研究为有毒含重金属废水和危险固体废物的同步处理提供了新思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号