当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
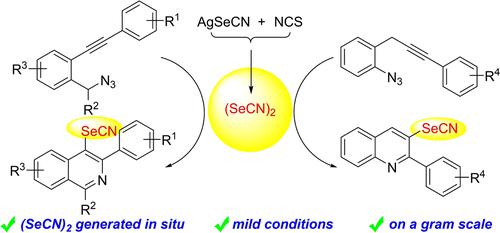
Synthesis of Isoquinolylselenocyanates and Quinolylselenocyanates via Electrophilic Selenocyanogen Cyclization Induced by Pseudohalogen (SeCN)2 Generated in situ
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-10-22 , DOI: 10.1002/adsc.202101169 Jia Wang 1 , Yun-Zhe Wang 2 , Yu-Jie Liu 1 , Xin-Xin Yan 1 , Yun-Hui Yan 1 , Shu-Jun Chao 1 , Xuefang Shang 1 , Tianjun Ni 1 , Ping-xin Zhou 3
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-10-22 , DOI: 10.1002/adsc.202101169 Jia Wang 1 , Yun-Zhe Wang 2 , Yu-Jie Liu 1 , Xin-Xin Yan 1 , Yun-Hui Yan 1 , Shu-Jun Chao 1 , Xuefang Shang 1 , Tianjun Ni 1 , Ping-xin Zhou 3
Affiliation
|
A strategy for the synthesis of isoquinolylselenocyanates and quinolylselenocyanates through electrophilic selenocyanogen cyclization has been developed. The feature of this reaction is that the sequential process was induced directly by generated in situ pseudohalogen (SeCN)2 generated in situ. Additionally, the obtained selenocyanates allowed functional group diversification, which could be potential intermediates for valuable compounds.
中文翻译:

通过原位产生的伪卤素 (SeCN)2 诱导亲电硒氰环化合成异喹啉基硒氰酸酯和喹啉硒氰酸酯
已经开发了通过亲电子硒氰基环化合成异喹啉基硒氰酸酯和喹啉基硒氰酸酯的策略。该反应的特征是,连续的过程直接诱导产生原位拟(SECN)2产生的原位。此外,获得的硒氰酸酯允许官能团多样化,这可能是有价值化合物的潜在中间体。
更新日期:2021-10-22
中文翻译:

通过原位产生的伪卤素 (SeCN)2 诱导亲电硒氰环化合成异喹啉基硒氰酸酯和喹啉硒氰酸酯
已经开发了通过亲电子硒氰基环化合成异喹啉基硒氰酸酯和喹啉基硒氰酸酯的策略。该反应的特征是,连续的过程直接诱导产生原位拟(SECN)2产生的原位。此外,获得的硒氰酸酯允许官能团多样化,这可能是有价值化合物的潜在中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号