当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous Cleavage at Glu and Gln Residues in Long-Lived Proteins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-22 , DOI: 10.1021/acschembio.1c00379 Michael G Friedrich 1 , Zhen Wang 2 , Kevin L Schey 2 , Roger J W Truscott 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-22 , DOI: 10.1021/acschembio.1c00379 Michael G Friedrich 1 , Zhen Wang 2 , Kevin L Schey 2 , Roger J W Truscott 1
Affiliation
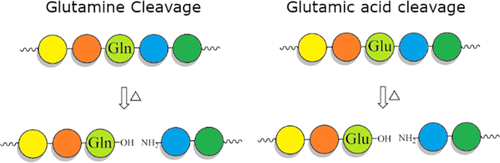
|
Long-lived proteins (LLPs) are prone to deterioration with time, and one prominent breakdown process is the scission of peptide bonds. These cleavages can either be enzymatic or spontaneous. In this study, human lens proteins were examined and many were found to have been cleaved on the C-terminal side of Glu and Gln residues. Such cleavages could be reproduced experimentally by in vitro incubation of Glu- or Gln-containing peptides at physiological pHs. Spontaneous cleavage was dependent on pH and amino acid sequence. These model peptide studies suggested that the mechanism involves a cyclic intermediate and is therefore analogous to that characterized for cleavage of peptide bonds adjacent to Asp and Asn residues. An increased amount of some Glu/Gln cleaved peptides in the insoluble fraction of human lenses suggests that cleavage may act to destabilize proteins. Spontaneous cleavage at Glu and Gln, as well as recently described cross-linking at these residues, can therefore be added to the similar processes affecting long-lived proteins that have already been documented for Asn and Asp residues.
中文翻译:

长寿命蛋白质中 Glu 和 Gln 残基的自发裂解
长寿命蛋白质 (LLP) 容易随着时间的推移而变质,其中一个突出的分解过程是肽键的断裂。这些裂解可以是酶促的或自发的。在这项研究中,检查了人晶状体蛋白,发现许多蛋白在 Glu 和 Gln 残基的 C 端侧被切割。这种裂解可以通过体外实验重现在生理 pH 条件下孵育含 Glu 或 Gln 的肽。自发裂解取决于 pH 值和氨基酸序列。这些模型肽研究表明该机制涉及环状中间体,因此类似于以裂解与 Asp 和 Asn 残基相邻的肽键为特征的机制。人晶状体的不溶性部分中一些 Glu/Gln 裂解肽的数量增加表明裂解可能会破坏蛋白质的稳定性。Glu 和 Gln 的自发裂解,以及最近描述的这些残基的交联,因此可以添加到影响长寿命蛋白质的类似过程中,这些过程已经被记录为 Asn 和 Asp 残基。
更新日期:2021-11-19
中文翻译:

长寿命蛋白质中 Glu 和 Gln 残基的自发裂解
长寿命蛋白质 (LLP) 容易随着时间的推移而变质,其中一个突出的分解过程是肽键的断裂。这些裂解可以是酶促的或自发的。在这项研究中,检查了人晶状体蛋白,发现许多蛋白在 Glu 和 Gln 残基的 C 端侧被切割。这种裂解可以通过体外实验重现在生理 pH 条件下孵育含 Glu 或 Gln 的肽。自发裂解取决于 pH 值和氨基酸序列。这些模型肽研究表明该机制涉及环状中间体,因此类似于以裂解与 Asp 和 Asn 残基相邻的肽键为特征的机制。人晶状体的不溶性部分中一些 Glu/Gln 裂解肽的数量增加表明裂解可能会破坏蛋白质的稳定性。Glu 和 Gln 的自发裂解,以及最近描述的这些残基的交联,因此可以添加到影响长寿命蛋白质的类似过程中,这些过程已经被记录为 Asn 和 Asp 残基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号