当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physicochemical and electrochemical characterization of salt-in-water and water-in-salt potassium and lithium acetate electrolytes
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ta07214e Mona Amiri 1 , Daniel Bélanger 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ta07214e Mona Amiri 1 , Daniel Bélanger 1
Affiliation
|
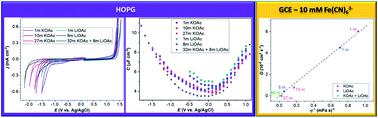
We report the physicochemical and electrochemical properties of various concentrations of potassium and lithium acetate (OAc) electrolytes from dilute to near saturation as well as mixed potassium/lithium acetate (32 m KOAc + 8 m LiOAc). The electrochemical stability window varied in the order of Pt < Au < highly ordered pyrolytic graphite (HOPG) < glassy carbon electrodes, and expanded when the salt concentration increased reaching 3.5 V in the dual cation water-in-salt electrolyte. This increase was due to the shift in the onset potential of the hydrogen evolution and oxygen evolution reactions to more negative and positive potential values, respectively. It is noteworthy that there is a larger shift in the onset potential of the oxygen evolution on the Pt electrode. The capacitance at the potential of zero charge of the HOPG electrode was found to be about 4 μF cm−2 and almost independent of the electrolyte concentration while the potential of zero charge shifted to more negative values upon increasing the acetate salt concentration. Using ferricyanide and ferrocene methanol as redox probes, their redox potential, ion transport and electron transfer kinetics in acetate-based electrolytes were investigated. The apparent redox potential shifted in the opposite direction; to more positive and negative values for ferricyanide and ferrocene methanol redox systems, respectively. A significant decrease in the diffusion coefficient of ferricyanide ions, i.e. 4 orders of magnitude, was observed in the dual cation water-in-salt compared to a dilute single salt electrolyte. High viscosities might be partially responsible for the decrease of the heterogeneous rate constants obtained for the redox probes but other factors such as interactions with water, electrolyte ions and electrodes might be at play as well.
中文翻译:

水包盐和盐包水钾和醋酸锂电解质的物理化学和电化学表征
我们报告了从稀释到接近饱和的各种浓度的醋酸钾和醋酸锂 (OAc) 电解质以及混合醋酸钾/醋酸锂 (32 m KOAc + 8 m LiOAc) 的物理化学和电化学特性。电化学稳定性窗口按照 Pt < Au < 高度有序热解石墨 (HOPG) < 玻碳电极的顺序变化,并在双阳离子盐包水电解质中盐浓度增加至 3.5 V 时扩大。这种增加是由于析氢和析氧反应的起始电位分别向更负和更正的电位值转变。值得注意的是,Pt 电极上析氧的起始电位有较大的变化。-2并且几乎与电解质浓度无关,而随着乙酸盐浓度的增加,零电荷电位转移到更负的值。使用铁氰化物和二茂铁甲醇作为氧化还原探针,研究了它们在乙酸盐基电解质中的氧化还原电位、离子传输和电子转移动力学。表观氧化还原电位向相反方向移动;分别为铁氰化物和二茂铁甲醇氧化还原系统的更多正值和负值。铁氰化物离子的扩散系数显着降低,即与稀释的单盐电解质相比,在双阳离子盐包水中观察到 4 个数量级。高粘度可能是氧化还原探针获得的异质速率常数降低的部分原因,但其他因素如与水、电解质离子和电极的相互作用也可能起作用。
更新日期:2021-10-22
中文翻译:

水包盐和盐包水钾和醋酸锂电解质的物理化学和电化学表征
我们报告了从稀释到接近饱和的各种浓度的醋酸钾和醋酸锂 (OAc) 电解质以及混合醋酸钾/醋酸锂 (32 m KOAc + 8 m LiOAc) 的物理化学和电化学特性。电化学稳定性窗口按照 Pt < Au < 高度有序热解石墨 (HOPG) < 玻碳电极的顺序变化,并在双阳离子盐包水电解质中盐浓度增加至 3.5 V 时扩大。这种增加是由于析氢和析氧反应的起始电位分别向更负和更正的电位值转变。值得注意的是,Pt 电极上析氧的起始电位有较大的变化。-2并且几乎与电解质浓度无关,而随着乙酸盐浓度的增加,零电荷电位转移到更负的值。使用铁氰化物和二茂铁甲醇作为氧化还原探针,研究了它们在乙酸盐基电解质中的氧化还原电位、离子传输和电子转移动力学。表观氧化还原电位向相反方向移动;分别为铁氰化物和二茂铁甲醇氧化还原系统的更多正值和负值。铁氰化物离子的扩散系数显着降低,即与稀释的单盐电解质相比,在双阳离子盐包水中观察到 4 个数量级。高粘度可能是氧化还原探针获得的异质速率常数降低的部分原因,但其他因素如与水、电解质离子和电极的相互作用也可能起作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号