Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sub-Nanometer Confined Ions and Solvent Molecules Intercalation Capacitance in Microslits of 2D Materials
Small ( IF 13.0 ) Pub Date : 2021-10-22 , DOI: 10.1002/smll.202104649 Yaqing Guo 1 , Xufeng Hong 2 , Yaqiong Su 3, 4 , Wen Luo 2 , Ruohan Yu 2 , Jinsong Wu 2 , Emiel J M Hensen 3 , Liqiang Mai 2 , Yuancheng Cao 5
Small ( IF 13.0 ) Pub Date : 2021-10-22 , DOI: 10.1002/smll.202104649 Yaqing Guo 1 , Xufeng Hong 2 , Yaqiong Su 3, 4 , Wen Luo 2 , Ruohan Yu 2 , Jinsong Wu 2 , Emiel J M Hensen 3 , Liqiang Mai 2 , Yuancheng Cao 5
Affiliation
|
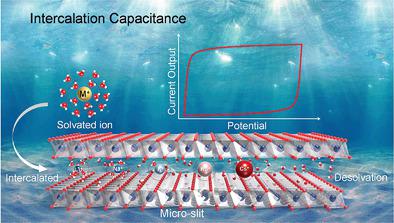
The ion intercalation behavior in 2D materials is widely applied in energy storage, electrocatalysis, and desalination. However, the detailed effect of ions on the performance, combining the influence of interlayer force and the change of solvent shell, is far less well understood. Here the solvated alkali metal ions with different sizes are intercalated into the lattice of 2D materials with different spacings (Ti3C2Tx, δ-MnO2, and reduced graphene oxide) to construct the intercalation model related with sub-nanometer confined ions and solvent molecules to further understand the intercalation capacitance. Based on electrochemical methods and density functional theory calculation, the ions lose the electrostatic shielding solvent shell or shorten the distance between the layers, resulting in a significant increase in capacitance. It is found that the intercalation capacitance arises from the diffusion of solvated ions and is controlled by quantum and electrochemical capacitance for desolvated ions. This effect of solvation structure on performance can be applied in a variety of electrochemical interface studies and provides a new research view for energy storage mechanisms.
中文翻译:

亚纳米受限离子和溶剂分子在二维材料微缝中的插层电容
二维材料中的离子嵌入行为广泛应用于储能、电催化和海水淡化。然而,离子对性能的详细影响,结合层间力的影响和溶剂壳的变化,远未得到很好的理解。在这里,不同尺寸的溶剂化碱金属离子嵌入不同间距的二维材料(Ti 3 C 2 T x,δ-MnO 2和还原氧化石墨烯)构建与亚纳米限制离子和溶剂分子相关的插层模型,以进一步了解插层电容。基于电化学方法和密度泛函理论计算,离子失去静电屏蔽溶剂壳或缩短层间距离,导致电容显着增加。发现嵌入电容由溶剂化离子的扩散产生,并受去溶剂化离子的量子和电化学电容控制。这种溶剂化结构对性能的影响可应用于多种电化学界面研究,为储能机制提供了新的研究视角。
更新日期:2021-12-09
中文翻译:

亚纳米受限离子和溶剂分子在二维材料微缝中的插层电容
二维材料中的离子嵌入行为广泛应用于储能、电催化和海水淡化。然而,离子对性能的详细影响,结合层间力的影响和溶剂壳的变化,远未得到很好的理解。在这里,不同尺寸的溶剂化碱金属离子嵌入不同间距的二维材料(Ti 3 C 2 T x,δ-MnO 2和还原氧化石墨烯)构建与亚纳米限制离子和溶剂分子相关的插层模型,以进一步了解插层电容。基于电化学方法和密度泛函理论计算,离子失去静电屏蔽溶剂壳或缩短层间距离,导致电容显着增加。发现嵌入电容由溶剂化离子的扩散产生,并受去溶剂化离子的量子和电化学电容控制。这种溶剂化结构对性能的影响可应用于多种电化学界面研究,为储能机制提供了新的研究视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号