Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bio-Derived Surface Layer Suitable for Long Term Cycling Ni-Rich Cathode for Lithium-Ion Batteries
Small ( IF 13.0 ) Pub Date : 2021-10-22 , DOI: 10.1002/smll.202104532 Chang-Heum Jo 1 , Natalia Voronina 1 , Hee Jae Kim 1 , Hitoshi Yashiro 2 , Najma Yaqoob 3 , Olivier Guillon 3 , Payam Kaghazchi 3 , Seung-Taek Myung 1
Small ( IF 13.0 ) Pub Date : 2021-10-22 , DOI: 10.1002/smll.202104532 Chang-Heum Jo 1 , Natalia Voronina 1 , Hee Jae Kim 1 , Hitoshi Yashiro 2 , Najma Yaqoob 3 , Olivier Guillon 3 , Payam Kaghazchi 3 , Seung-Taek Myung 1
Affiliation
|
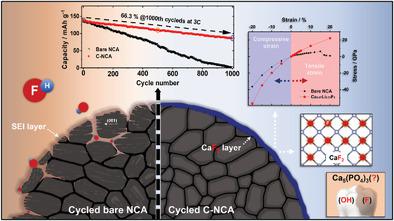
Since Ni-rich cathode material is very sensitive to moisture and easily forms residual lithium compounds that degrade cell performance, it is very important to pay attention to the selection of the surface modifying media. Accordingly, hydroxyapatite (Ca5(PO4)3(OH)), a tooth-derived material showing excellent mechanical and thermodynamic stabilities, is selected. To verify the availability of hydroxyapatite as a surface protection material, lithium-doped hydroxyapatite, Ca4.67Li0.33(PO4)3(OH), is formed with ≈10-nm layer after reacting with residual lithium compounds on Li[Ni0.8Co0.15Al0.05]O2, which spontaneously results in dramatic reduction of surface lithium residues to 2879 ppm from 22364 ppm. The Ca4.67Li0.33(PO4)3(OH)-modified Li[Ni0.8Co0.15Al0.05]O2 electrode provides ultra-long term cycling stability, enabling 1000 cycles retaining 66.3% of its initial capacity. Also, morphological degradations such as micro-cracking or amorphization of surface are significantly suppressed by the presence of Ca4.67Li0.33(PO4)3(OH) layer on the Li[Ni0.8Co0.15Al0.05]O2, of which the Ca4.67Li0.33(PO4)3(OH) is transformed to CaF2 via Ca4.67Li0.33(PO4)3F during the long term cycles reacting with HF in electrolyte. In addition, the authors’ density function theory (DFT) results explain the reason of instability of NCA and why CaF2 layers can delay the micro-cracking during electrochemical reaction. Therefore, the stable Ca4.67Li0.33(PO4)3F and CaF2 layers play a pivotal role to protect the Li[Ni0.8Co0.15Al0.05]O2 with ultra-long cycling stability.
中文翻译:

适用于锂离子电池的长期循环富镍阴极的生物衍生表面层
由于富镍正极材料对水分非常敏感,容易形成残留的锂化合物,降低电池性能,因此注意表面改性介质的选择非常重要。因此,选择羟基磷灰石(Ca 5 (PO 4 ) 3 (OH)),一种表现出优异机械和热力学稳定性的牙齿衍生材料。为了验证羟基磷灰石作为表面保护材料的有效性,在与 Li[Ni 0.8 Co上的残留锂化合物反应后,掺杂锂的羟基磷灰石 Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 形成了约 10 纳米的层0.15铝0.05]O 2,这会自发地导致表面锂残留量从 22364 ppm 急剧减少到 2879 ppm。Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 改性的 Li[Ni 0.8 Co 0.15 Al 0.05 ]O 2电极提供超长期循环稳定性,使 1000 次循环保持其初始容量的 66.3%。此外,在 Li[Ni 0.8 Co 0.15上存在 Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 层,显着抑制了表面的微裂纹或非晶化等形态退化Al 0.05 ]O 2,其中Ca 4.67 Li 0.33 (PO 4 ) 3 (OH)在长期循环中与电解液中的HF反应,通过Ca 4.67 Li 0.33 (PO 4 ) 3 F转化为CaF 2。此外,作者的密度函数理论 (DFT) 结果解释了 NCA 不稳定的原因以及为什么 CaF 2层可以延迟电化学反应过程中的微裂纹。因此,稳定的 Ca 4.67 Li 0.33 (PO 4 ) 3 F 和 CaF 2层在保护具有超长循环稳定性的 Li[Ni 0.8 Co 0.15 Al 0.05 ]O 2方面发挥着关键作用。
更新日期:2021-11-25
中文翻译:

适用于锂离子电池的长期循环富镍阴极的生物衍生表面层
由于富镍正极材料对水分非常敏感,容易形成残留的锂化合物,降低电池性能,因此注意表面改性介质的选择非常重要。因此,选择羟基磷灰石(Ca 5 (PO 4 ) 3 (OH)),一种表现出优异机械和热力学稳定性的牙齿衍生材料。为了验证羟基磷灰石作为表面保护材料的有效性,在与 Li[Ni 0.8 Co上的残留锂化合物反应后,掺杂锂的羟基磷灰石 Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 形成了约 10 纳米的层0.15铝0.05]O 2,这会自发地导致表面锂残留量从 22364 ppm 急剧减少到 2879 ppm。Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 改性的 Li[Ni 0.8 Co 0.15 Al 0.05 ]O 2电极提供超长期循环稳定性,使 1000 次循环保持其初始容量的 66.3%。此外,在 Li[Ni 0.8 Co 0.15上存在 Ca 4.67 Li 0.33 (PO 4 ) 3 (OH) 层,显着抑制了表面的微裂纹或非晶化等形态退化Al 0.05 ]O 2,其中Ca 4.67 Li 0.33 (PO 4 ) 3 (OH)在长期循环中与电解液中的HF反应,通过Ca 4.67 Li 0.33 (PO 4 ) 3 F转化为CaF 2。此外,作者的密度函数理论 (DFT) 结果解释了 NCA 不稳定的原因以及为什么 CaF 2层可以延迟电化学反应过程中的微裂纹。因此,稳定的 Ca 4.67 Li 0.33 (PO 4 ) 3 F 和 CaF 2层在保护具有超长循环稳定性的 Li[Ni 0.8 Co 0.15 Al 0.05 ]O 2方面发挥着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号