Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2021-10-22 , DOI: 10.1016/j.jhazmat.2021.127584 Sicong Lei 1 , Chengyi Hong 2 , Zhiqiang Dong 3 , Jichen Zhang 2 , Xiaoxian Zhang 2 , Ling Zhu 2 , Yuping Qiu 4
|
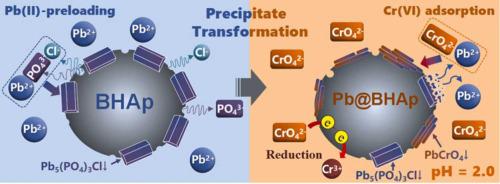
In this work, the mechanism of Pb(II)-mediated precipitation transformation to improve the removal of Cr(VI)-oxyanion on biogenic hydroxyapatite (BHAp) were investigated. The Pb(II)-preloading formed pyromorphite [Pb5(PO4)3Cl] precipitate on the BHAp surface (Pb@BHAp), thus causing an increase of 2.2 times in the uptake of Cr(VI) by Pb@BHAp at pH of 2.4. It was primarily due to the dissolution of Pb5(PO4)3Cl accompanied with the release of Pb(II), resulting in the rapid formation of crocoite (PbCrO4). Although the Ksp of Pb5(PO4)3Cl was approximately 23 orders of magnitude lower than that of PbCrO4, Pb(II)-mediated precipitation transformation could still occur. XRD and SEM-EDX analyses demonstrated that the process was a time-dependent that included rapid crystal precipitation in the initial 10 min and subsequent precipitate accumulation for several hours. The Pb(II) released from the dissolution of Pb5(PO4)3Cl was immediately immobilized by Cr(VI); therefore, it did not cause any retention risk of Pb(II) in the solution. Furthermore, a small quantity of Cr(VI) could be reduced to Cr(III) by BHAp, and Cr(III) could enter into the BHAp lattice for the exchange of Ca(II). This study provides a new insight into the resource utilization of Pb-bearing BHAp and a potential method for the successive removal of Pb(II) and Cr(VI).
中文翻译:

Pb(II) 介导的沉淀转化促进生物源羟基磷灰石对 Cr(VI) 的固定化
在这项工作中,研究了 Pb(II) 介导的沉淀转化改善生物羟基磷灰石 (BHAp) 上 Cr(VI)-氧阴离子去除的机制。Pb(II)-预加载在 BHAp 表面形成焦极铁矿 [Pb 5 (PO 4 ) 3 Cl] (Pb@BHAp),从而导致 Pb@BHAp 对 Cr(VI) 的吸收增加了 2.2 倍pH 值为 2.4。这主要是由于Pb 5 (PO 4 ) 3 Cl 的溶解伴随着Pb(II)的释放,导致铬铅矿(PbCrO 4 )的快速形成。虽然Pb 5 (PO 4 ) 3的K spCl比PbCrO 4低约23个数量级,Pb(II)介导的沉淀转变仍可能发生。XRD 和 SEM-EDX 分析表明,该过程是时间依赖性的,包括最初 10 分钟内的快速晶体沉淀和随后几个小时的沉淀物积累。Pb 5 (PO 4 ) 3溶解释放的Pb(II)Cl 立即被 Cr(VI) 固定;因此,它不会导致溶液中 Pb(II) 的任何保留风险。此外,少量的Cr(VI)可以被BHAp还原为Cr(III),Cr(III)可以进入BHAp晶格进行Ca(II)的交换。该研究为含铅 BHAp 的资源利用提供了新的见解,并为连续去除 Pb(II) 和 Cr(VI) 提供了一种潜在的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号