当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Structure-Based Optimization of Fragments Binding the Mixed Lineage Kinase Domain-like Protein Executioner Domain
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-21 , DOI: 10.1021/acs.jmedchem.1c00686 Martin Rübbelke 1 , James Hamilton 1 , Florian Binder 1 , Margit Bauer 1 , Jim King 2 , Herbert Nar 1 , Markus Zeeb 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-21 , DOI: 10.1021/acs.jmedchem.1c00686 Martin Rübbelke 1 , James Hamilton 1 , Florian Binder 1 , Margit Bauer 1 , Jim King 2 , Herbert Nar 1 , Markus Zeeb 1
Affiliation
|
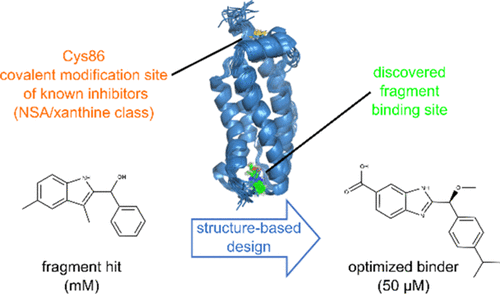
Necroptosis is a form of programmed cell death that in case of misregulation can lead to inflammatory diseases. Mixed lineage kinase domain-like protein (MLKL), the effector protein in the canonical necroptosis signaling pathway, becomes activated by phosphorylation. Here, we report the identification of novel reversible binders of the MLKL executioner domain by a protein NMR-detected fragment-based screen. Determination of protein fragment costructures using NMR spectroscopy revealed a small molecule binding site that is distinct from the previously identified binding site of covalent MLKL inhibitors. Affinity optimization of the initially prioritized hit with millimolar affinity was achieved by NMR-guided structure-based design and yielded fragment-like molecules with a KD of 50 μM. Furthermore, we demonstrate that the improved fragment competes for the same binding site as nonyl-maltoside, a detergent that in conjunction with phytic acid activates the MLKL executioner domain.
中文翻译:

结合混合谱系激酶域样蛋白执行域的片段的发现和基于结构的优化
坏死性凋亡是程序性细胞死亡的一种形式,如果调节不当会导致炎症性疾病。混合谱系激酶结构域样蛋白 (MLKL),经典坏死性凋亡信号通路中的效应蛋白,通过磷酸化被激活。在这里,我们报告了通过蛋白质 NMR 检测的基于片段的屏幕识别 MLKL 刽子手域的新型可逆结合剂。使用 NMR 光谱测定蛋白质片段共结构揭示了一个小分子结合位点,该位点不同于先前确定的共价 MLKL 抑制剂的结合位点。通过 NMR 引导的基于结构的设计实现了初始优先命中与毫摩尔亲和力的亲和力优化,并产生了具有K D 的片段样分子50 μM。此外,我们证明改进的片段与壬基麦芽糖苷竞争相同的结合位点,壬基麦芽糖苷是一种去污剂,与植酸结合激活 MLKL 刽子手结构域。
更新日期:2021-11-11
中文翻译:

结合混合谱系激酶域样蛋白执行域的片段的发现和基于结构的优化
坏死性凋亡是程序性细胞死亡的一种形式,如果调节不当会导致炎症性疾病。混合谱系激酶结构域样蛋白 (MLKL),经典坏死性凋亡信号通路中的效应蛋白,通过磷酸化被激活。在这里,我们报告了通过蛋白质 NMR 检测的基于片段的屏幕识别 MLKL 刽子手域的新型可逆结合剂。使用 NMR 光谱测定蛋白质片段共结构揭示了一个小分子结合位点,该位点不同于先前确定的共价 MLKL 抑制剂的结合位点。通过 NMR 引导的基于结构的设计实现了初始优先命中与毫摩尔亲和力的亲和力优化,并产生了具有K D 的片段样分子50 μM。此外,我们证明改进的片段与壬基麦芽糖苷竞争相同的结合位点,壬基麦芽糖苷是一种去污剂,与植酸结合激活 MLKL 刽子手结构域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号