Water Research ( IF 11.4 ) Pub Date : 2021-10-20 , DOI: 10.1016/j.watres.2021.117787 Quanzhen Liu 1 , Xiong Xu 2 , Jianjie Fu 3 , Yanjun Du 1 , Lihua Lin 2 , Lu Bai 1 , Donghong Wang 2
|
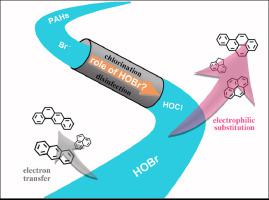
Hypobromous acid (HOBr), a highly reactive active species, can be formed and impact reaction processes with organic pollutants in source water during chlorination disinfection of the water containing bromide (Brˉ). In this study, we investigated the transformation kinetics of 10 parent polycyclic aromatic hydrocarbons (PAHs) and formation mechanisms of transformation products in the presence of Brˉ during chlorination. The results indicated that HOBr can accelerate the processes of electrophilic substitution (ES) and single electron transfer (ET) reactions in PAHs, and the second-order rate constants of HOBr are 102–103 times higher than those of hypochlorous acid (HOCl) with PAHs. HOBr was more conductive to induce ES reactions than HOCl. In water containing Brˉ, HOBr and HOCl dominate the reaction processes with PAHs, although other active bromine species may still affect reaction processes. In terms of transformation products, higher reactivity of HOBr results from faster formation of oxygenated PAHs (OPAHs) and halogenated PAHs (HPAHs) than HOCl. As an example of 3 model PAHs, anthracene transforms faster to its oxygenated products at a higher concentration, while pyrene and fluorene transform faster to halogenated products. These fundamental results were essential to understanding the transformation kinetics of PAHs and the formation of toxic disinfection by-products in the presence of Brˉ.
中文翻译:

次溴酸在氯化多环芳烃转化中的作用
次溴酸 (HOBr) 是一种高活性活性物质,在对含溴化物 (Brˉ) 的水进行氯化消毒过程中,会形成并影响与源水中有机污染物的反应过程。在本研究中,我们研究了氯化过程中在 Brˉ 存在下 10 种母体多环芳烃 (PAH) 的转化动力学和转化产物的形成机制。结果表明HOBr可以加速PAHs中的亲电取代(ES)和单电子转移(ET)反应过程,HOBr的二级速率常数为10 2 –10 3比含有多环芳烃的次氯酸 (HOCl) 高出数倍。HOBr 比 HOCl 更能诱导 ES 反应。在含有 Brˉ 的水中,HOBr 和 HOCl 在与 PAHs 的反应过程中占主导地位,尽管其他活性溴物质仍可能影响反应过程。在转化产物方面,与 HOCl 相比,HOBr 的更高反应性是由于氧化多环芳烃 (OPAH) 和卤化多环芳烃 (HPAH) 的形成速度更快。作为 3 个模型 PAH 的示例,蒽在较高浓度下更快地转化为其含氧产物,而芘和芴更快地转化为卤化产物。这些基本结果对于理解多环芳烃的转化动力学和在 Brˉ 存在下有毒消毒副产物的形成至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号