当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Hydrolysis of Atmospherically Relevant Monoterpene-Derived Organic Nitrates
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-10-20 , DOI: 10.1021/acs.est.1c05310 Yuchen Wang 1 , Ivan R Piletic 2 , Masayuki Takeuchi 3 , Tianchang Xu 1 , Stefan France 4 , Nga Lee Ng 1, 3, 5
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-10-20 , DOI: 10.1021/acs.est.1c05310 Yuchen Wang 1 , Ivan R Piletic 2 , Masayuki Takeuchi 3 , Tianchang Xu 1 , Stefan France 4 , Nga Lee Ng 1, 3, 5
Affiliation
|
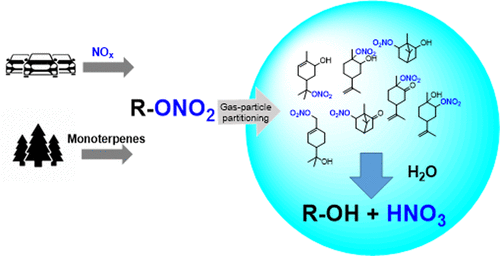
The partition of gas-phase organic nitrates (ONs) to aerosols and subsequent hydrolysis are regarded as important loss mechanisms for ON species. However, the hydrolysis mechanisms and the major factors controlling the hydrolysis lifetime are not fully understood. In this work, we synthesized seven monoterpene-derived ONs and systematically investigated their hydrolysis in bulk solutions at different pH values. The hydrolysis lifetimes ranged from 12.9 min to 8.5 h for allylic primary ON and tertiary ONs, but secondary ONs were stable at neutral pH. The alkyl substitution numbers, functional groups, and carbon skeletons were three important factors controlling hydrolysis rates. Tertiary and secondary ONs were found to hydrolyze via the acid-catalyzed unimolecular (SN1) mechanism, while a competition of SN1 and bimolecular (SN2) mechanisms accounted for the hydrolysis of primary ONs. The consistency of experimental and theoretical hydrolysis rates calculated by density functional theory further supported the proposed mechanisms. Reversible reactions including hydrolysis and nitration were first reported to explain the hydrolysis of ONs, highlighting the possibility that particulate nitric acid can participate in nitration to generate new nitrogen-containing compounds. These findings demonstrate that ON hydrolysis is a complex reaction that proceeds via different mechanisms and is controlled by various parameters.
中文翻译:

与大气相关的单萜衍生有机硝酸盐的合成和水解
气相有机硝酸盐 (ON) 向气溶胶的分配和随后的水解被认为是 ON 物种的重要损失机制。然而,水解机理和控制水解寿命的主要因素尚不完全清楚。在这项工作中,我们合成了七种单萜衍生的 ONs,并系统地研究了它们在不同 pH 值的本体溶液中的水解。烯丙基一级 ON 和三级 ON 的水解寿命为 12.9 分钟至 8.5 小时,但二级 ON 在中性 pH 值下稳定。烷基取代数、官能团和碳骨架是控制水解速率的三个重要因素。发现三级和二级 ONs 通过酸催化的单分子 (S N 1) 机制水解,而 S 的竞争N 1 和双分子 (S N 2) 机制解释了初级 ONs 的水解。通过密度泛函理论计算的实验和理论水解速率的一致性进一步支持了所提出的机制。首先报道了包括水解和硝化在内的可逆反应来解释ON的水解,突出了颗粒硝酸可以参与硝化以产生新的含氮化合物的可能性。这些发现表明 ON 水解是一个复杂的反应,它通过不同的机制进行并受各种参数的控制。
更新日期:2021-11-02
中文翻译:

与大气相关的单萜衍生有机硝酸盐的合成和水解
气相有机硝酸盐 (ON) 向气溶胶的分配和随后的水解被认为是 ON 物种的重要损失机制。然而,水解机理和控制水解寿命的主要因素尚不完全清楚。在这项工作中,我们合成了七种单萜衍生的 ONs,并系统地研究了它们在不同 pH 值的本体溶液中的水解。烯丙基一级 ON 和三级 ON 的水解寿命为 12.9 分钟至 8.5 小时,但二级 ON 在中性 pH 值下稳定。烷基取代数、官能团和碳骨架是控制水解速率的三个重要因素。发现三级和二级 ONs 通过酸催化的单分子 (S N 1) 机制水解,而 S 的竞争N 1 和双分子 (S N 2) 机制解释了初级 ONs 的水解。通过密度泛函理论计算的实验和理论水解速率的一致性进一步支持了所提出的机制。首先报道了包括水解和硝化在内的可逆反应来解释ON的水解,突出了颗粒硝酸可以参与硝化以产生新的含氮化合物的可能性。这些发现表明 ON 水解是一个复杂的反应,它通过不同的机制进行并受各种参数的控制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号